Greetings to everyone! In my previous post we have discussed the trace components present in the natural gas. We have also studied removal methods of some trace components which are essential for the processing of natural gas. Today, we shall discuss particularly about Helium as I have mentioned in my previous post already.

WHAT WE SHALL LEARN?
We shall be learning about 3 main processes. This includes the recovery of helium, the upgradation of helium and purification of helium. We shall discuss all these 3 processes.
Uses and Occurrence of Helium
First let us see the uses of Helium. Helium is used as a coolant in the superconductor for MRI applications in the hospitals. Then it is used as particle accelerators and in high energy physics research. It has been used in the gas cooled nuclear power reactors. It is used as a carrier gas in analytical equipment such as gas chromatographs. The deep-sea divers uses helium/oxygen breathing gas mixture to avoid nitrogen narcosis.
Helium is generally very much abundant in the sun but on the earth we do not have much of it. In the atmosphere we only have about 5 ppmv helium. The viable source of helium is natural gas.
Helium Recovery
Now for the helium recovery, generally the dump from nitrogen rejection unit produces raw helium stream containing roughly an equimolar mixture of helium and nitrogen. If the LNG plant does not have a helium recovery unit, any He in the feed gas will be vented to atmosphere with the N2 vent stream.

Helium can be recovered by using cryogenic distillation which is a very conventional method but it is quite energy intensive. So it is quite expensive as well. Currently there is no alternative commercially available process technology. But some other technologies are being developed which are membrane based and pressure swing adsorption (PSA). They have large capital and energy-cost savings compared with cryogenic distillation processes. They are used in industrial applications like hydrogen production in chemical and petrochemical processes.
Helium Upgradation
In the upgradation process, we want to separate the nitrogen from the exit gas stream of the nitrogen recovery unit (NRU). The other impurities in the exit gas streams are methane, hydrogen, neon etc. For this we further cool it down at lower temperatures than the temperatures in the NRU by cryogenic distillation. This is done before the liquefaction. Then it is partially condensed to provide a N2 rich liquid stream which is warmed and separated to provide a waste stream that can be used to regenerate the downstream driers. A vapor stream of upgraded He contains approximately 90% He. This 90% benchmark is required to meet product specifications and to prevent the freezing out of impurities in the liquefaction process, because He has the lowest boiling among all other fluids.
Helium Purification
The upgraded He is then purified to achieve a purity of 99.995%. This is done by mixing the upgraded He with air which provides O2 for combustion of H2. It is then preheated to above 300K and passed through a catalyst bed to oxidize any trace H2 or remaining hydrocarbons. The reactor product is cooled to condense any water produced by combustion of the H2. It is drained out and dehydrated and made free from CO2 and O2 by adsorption with molecular sieves.

Adsorption Based He Recovery
Now let us have a look at how the adsorption is being carried out for He recovery. Well, this is PSA based and removes trace N2 from upgraded He to achieve 99.999% He product streams. All the other impurities are adsorbed because He generally has the lowest affinity for adsorption. This adsorption needs upgraded feed stream (90% He) and it generally uses zeolite and narrow pore activated carbons.
Membrane Separation for He Recovery
Next we come to membrane separation for He recovery. This process is introduced later than the adsorption in 1985. Here, the membranes exhibit high permeability for He compared with N2, methane and CO2 and other components of natural gas due to small size of He. These membranes are suitable for the coarse separation of bulk N2 and He. This is then followed by adsorption based He purification process. We now also have hybrid membrane cryogenic or membrane cryogenic adsorption processes which have been reported. Some of the membranes used for He recovery are -
- Ultra-microporous silica
- Molecular sieve carbons
- Titanium silicates
- Polyimide
The aromatic polyimide based membranes gives the highest selectivity of He with respect to both nitrogen and methane.
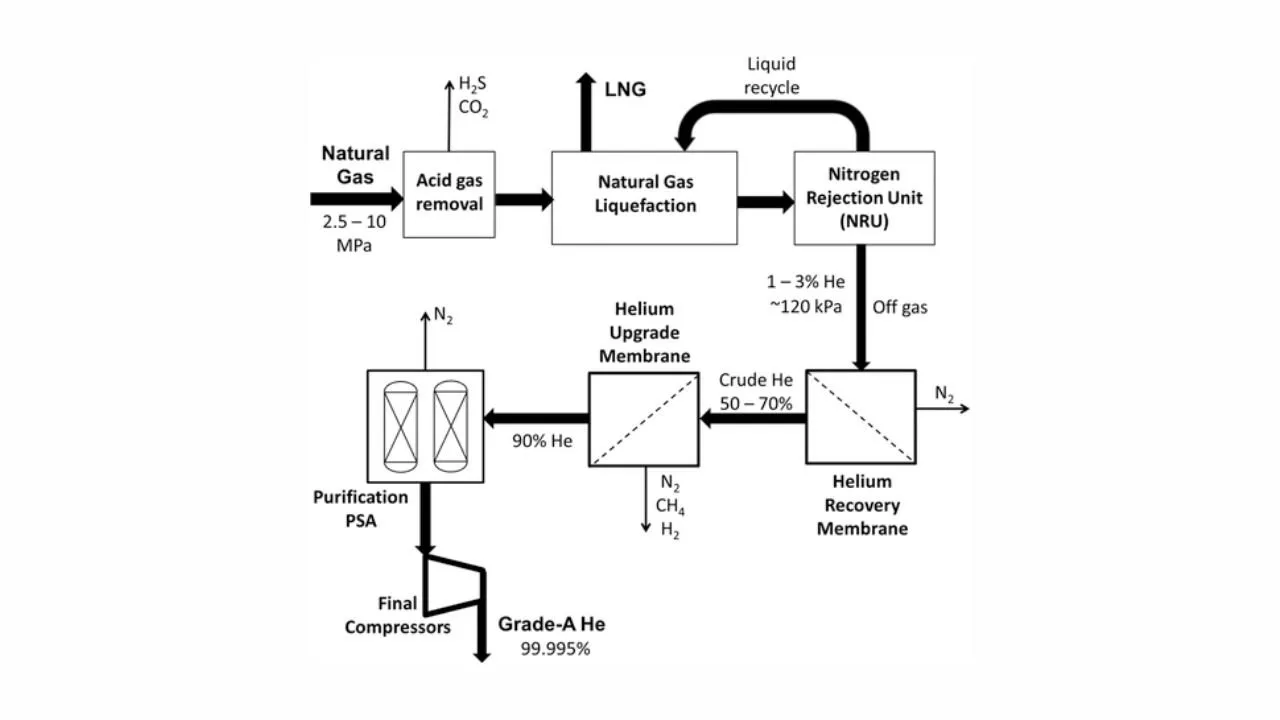
Industrial Membrane System for He Recovery
In the industrial membrane system recovery, we need to increase the purity of He. Several membrane stages can be operated in series in a two stage process that we have learnt earlier. With the passes of each membrane the purity of He increases. Due to the large pressure drop often around 1500-3000 kPa of the He rich permeate across the membrane unit, multistage membrane processes require inter-stage compressors. Whenever we use multistage, we need to compress as we should be having enough driving force for the separation through the membranes. That is why after each stage, we need to use a compressor for moving into the next stage.
Summary
We can say that the cryogenic distillation is the most commonly used process technology for He recovery. Adsorption is used in the He upgrading and purification stages and to remove any residual amount of N2 and H2. The recovery and purification processes of He are comparable in many ways with systems designed for hydrogen purification.
Recent technological advances for H2 separation from methane, nitrogen and carbon dioxide may be applicable to a He recovery process, which could allow quicker deployment of the technology.

Principle of Mass Transfer and Separation Process
Cryogenic Systems by R.F. Barron
Handbook of Natural Gas Transmission and Processing
Trace Components in Natural Gas System |ChemFam #24|
Sulphur Recovery in Natural Gas System-II |ChemFam #23|
Sulphur Recovery in Natural Gas System-I |ChemFam #22|
Nitrogen Removal in Natural Gas System-II |ChemFam #21|
Nitrogen Removal in Natural Gas System-I |ChemFam #20|
Acid Gas Removal in Natural Gas System-II |ChemFam #19|
Acid Gas Removal in Natural Gas System-I |ChemFam #18|
Estimation of Water Content in Natural Gas |ChemFam #17|
Membrane Separation in Natural Gas System |ChemFam #16|
Design of distillation column |ChemFam #15|
Separation Technique: Distillation |ChemFam #14|
Transmission Electron Microscope: Principle and Working |ChemFam #13|
Scanning Electron Microscope: Principle and Working |ChemFam #12|
Drugs: Classification and drug-target interaction |ChemFam #11|
What are orbitals and quantum numbers? |ChemFam #10|
Quantum mechanical model of an atom |ChemFam #09|
A case study about the growth mechanism of CNT |ChemFam #08|
Carbon Nanotubes (Buckytubes): Types and Synthesis |ChemFam #07|
Nanomaterials: Classification and Approach for Synthesis |ChemFam #06|
Azadirachtin: Isolation, Extraction and Mechanism of Action |ChemFam #05|
Woodward-Fieser Rules for Calculating λmax |ChemFam #04|
Chemistry in ancient India |ChemFam #03|
How do soaps clean the dirt? |ChemFam #02|
What is anti egg white injury factor? |ChemFam #01|
PS The thumbnail image is being created by me using canva.com taking template image from NYU


