In my previous post, I was talking about the quantum model of an atom. I gave a basic idea about the origin and a brief explanation of it. Today, I am going to write about orbitals and quantum numbers. Quantum numbers may be defined as a set of four numbers with the help of which we can give the complete information about all the electrons in an atom. It will not be wrong to say that they tell us the address of the electron i.e., location, energy, the type of orbital occupied and the orientation of that orbital. I have already talked about orbital.
The wave function ψ is found by solving the Schrödinger wave equation. An atomic orbital is found by wave function ψ for an electron in an atom. Whenever an electron is specified by a wave function, we say that the electron occupies that orbital. All the information about the electron is stored in the wave function. Since many wave functions are possible for an electron, there are many atomic orbitals in an atom. These ‘’one electron orbital wave functions” or ‘’orbitals” gives the basis of the electronic structure of atom.

There are four quantum numbers namely,
- Principal quantum number (n)
- Azimuthal quantum number (l)
- Magnetic orbital quantum number (ml)
- Electron spin quantum number (ms)
Each atomic orbital is designated by three quantum numbers labelled as n, l and ml. Let’s discuss each of them one by one.
This tells about the energy of shell, determines the size and energy of the orbital. There is always an positive integer value for n like n =1, 2, 3, …..
The following are the informations which are obtained from principal quantum number :
(i) It tells about the shell to which the electron belongs.
For example, electron with principal quantum number n = 1, belongs to the first shell. Similarly the electron with n = 3, belongs to the third shell around the nucleus.
Now these shells are also represented by letters.
| Principal Quantum Number (n) | Shell |
|---|---|
| 1 | 1st or K shell |
| 2 | 2nd or L shell |
| 3 | 3rdor M shell |
| 4 | 4th or N shell |
| So on…. | ….. |
(ii) It specifies the size of the shell.
For hydrogen atom or hydrogen-like species n value only determines the size of shell. With the increase in the principal quantum number (n), the size of the shell also increases.
This means as the principal quantum number increases, size of shell increases, therefore now the electron will be away from the nucleus.
(iii) It tells about the energy of the shell.
Principal quantum number alone determines the energy of the shell of hydrogen atom or hydrogen like-species. Therefore, larger the distance of the shell in which the electron is present, higher will be its energy.
(iv) It tells about the maximum number of electrons that a shell can accommodate is 2n2.
| Shell | K | L | M | N |
|---|---|---|---|---|
| Principal quantum number (n) | 1 | 2 | 3 | 4 |
| Maximum number of electrons | 2 | 8 | 18 | 32 |
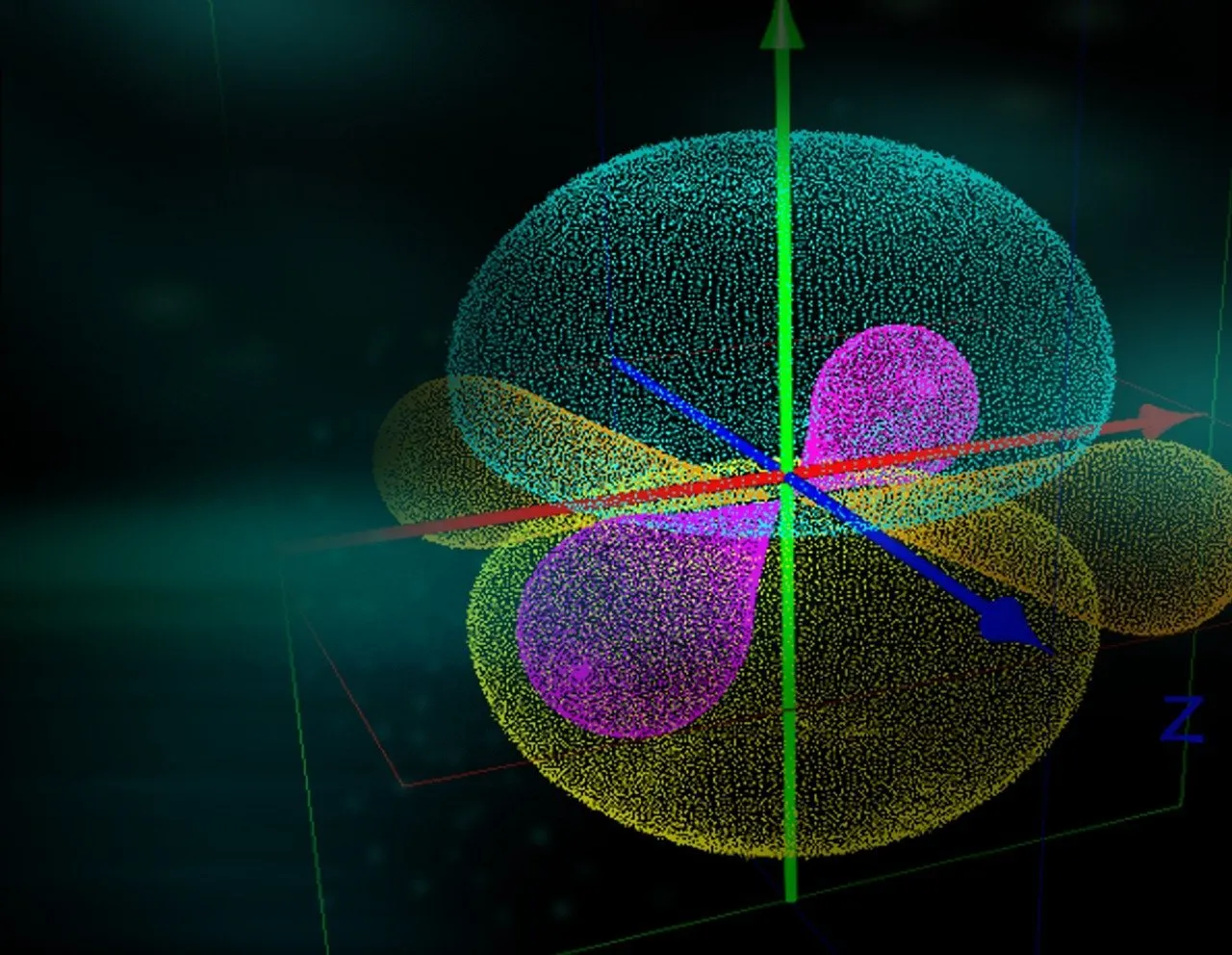
The other name for this quantum number is subsidiary quantum number which tells us about orbital angular momentum.
The following are the informations which are obtained from it-
(i) It designates the subshells to which the electron belongs.
(ii) It tells about the shape if orbitals.
For a given value of quantum number (n), l can have values ranging from 0 to n-1. If n value is 2, then values of l will be 0 to (n-1) i.e., 0 and 1. Moreover the number of subshells in a principal shell is equal to the value of n. e.g. there is only one subshell (l=0) in the first shell (n=1). The subshells corresponding to different values of l are represented by the following symbols.
| Value of l | Subshell notation | Shape of orbital |
|---|---|---|
| 0 | s | Spherical |
| 1 | p | Dumb-bell |
| 2 | d (except dz2) | Double dumb-bell |
| 3 | f | Complex |
The dz2 has baby soother like shape.
s, p, d, f are the symbols for sharp, principle, diffused, fundamental lines.
| n values | l values | Representation of subshell |
|---|---|---|
| 1 | 0 | 1s |
| 2 | 0, 1 | 2s, 2p |
| 3 | 0, 1, 2 | 3s, 3p, 3d |
| 4 | 0, 1, 2, 3 | 4s, 4p, 4d, 4f |
Angular quantum number of the electron in an orbital = √{l(l+1)} h/2π = √{l(l+1)} ℏ
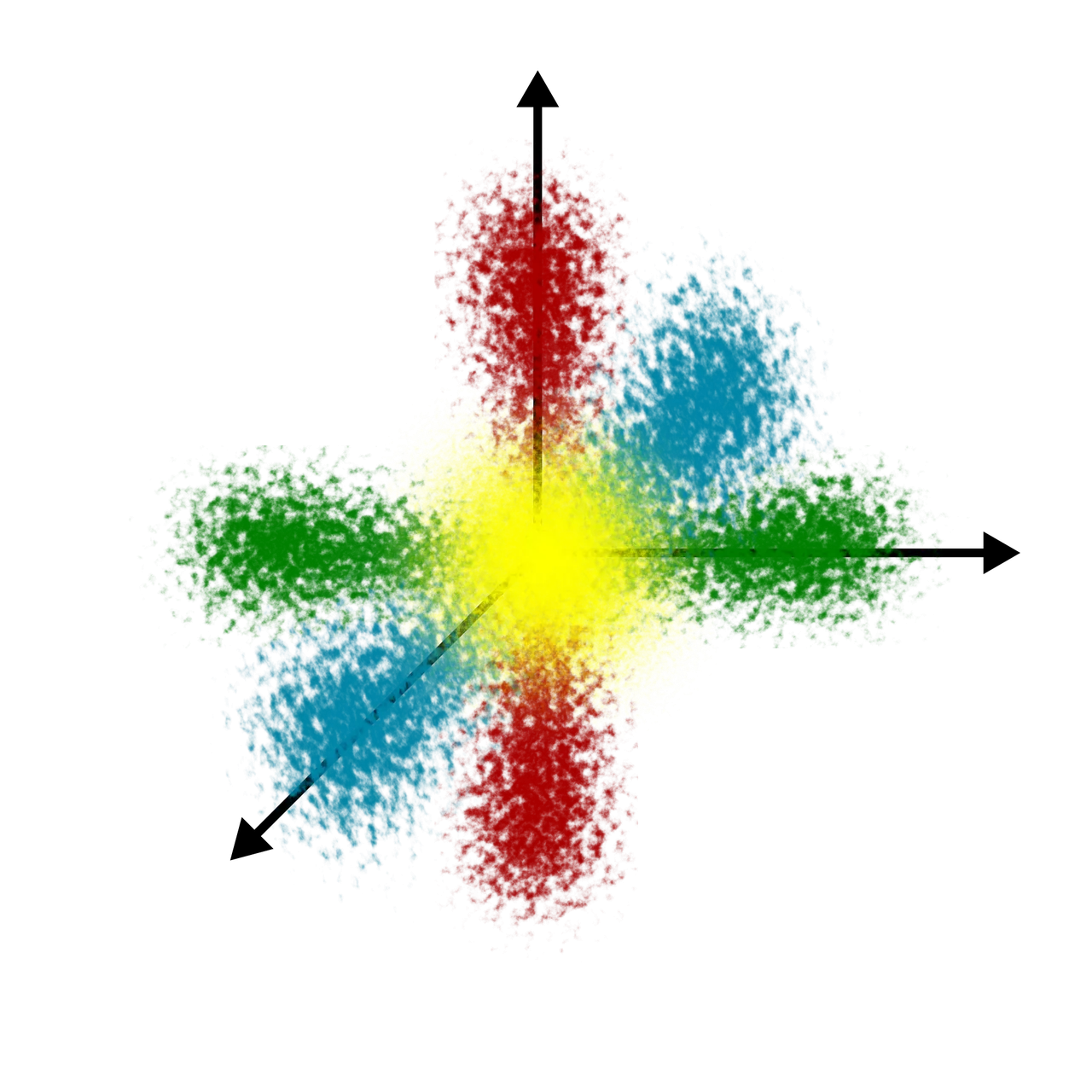
The magnetic orbital quantum number ml determines the number of preferred orientations of orbitals in a subshell. Also we can say that it gives the total number of orbitals present in a given subshell. As the name itself suggests this quantum number describes the behaviour of electron in a magnetic field. An electron revolving around the nucleus generates magnetic field. Under the influence of external magnetic field, the electrons of a subshell can orient themselves in certain preferred regions of space around the nucleus.
For a given subshell defined by l values there are 2l+1 values of ml which are possible.
Example :
For an electron belonging to l = 2 subshell, there are 2 l + 1 ml values i.e., (2 x 2 + 1) = 5 ml values.
Number of ml values gives the number of preferred orientations of the electron in a subshell or the number of orbitals in a subshell. Therefore, as there are 5 ml values, so 5 numbers of orbitals are present in l = 2 subshell or d subshell. Moreover the number of ml values ranges from +l to -l including 0. So, for l = 2, the ml values are, ml = +2, +1, 0, -1, -2. This means, d subshell contains five orbitals called d orbitals.
| Value of l | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Subshell | s | p | d | f | g |
| Number of orbitals | 1 | 3 | 5 | 7 | 9 |
The above three quantum number explains the energy, shape and orientations of orbitals. But it was observed with the help of an instrument of high resolving power that the spherical lines of some multi-electron atom occur in the form of doublets (two closely spaced lines), triplets (three closely spaced lines) etc. This suggested the presence of a few more energy levels than predicted till that time.
The presence of two closely spaced lines account to the fact these are the representation of the two different states of electrons. These states were later on identified as the spin of the electron. In 1925, George Uhlenbeck and Samuel Goudsmit proposed the fourth quantum number known as electron spin quantum number ms. The two scientists suggested that electron rotates on its own axis either in a clockwise or anti clockwise direction and therefore the spin quantum number is denoted by two values: +1/2 and -1/2 . The two values +1/2 and -1/2 are usually designated by ↑ (spin up) or ↓ (spin down) respectively. The two electrons that have different ms values ( +1/2 and -1/2 ) are said to have opposite spins. An orbital can hold maximum of two electrons with opposite spins.
Spin angular momentum of the electron = √{s(s+1)} h/2π = √{s(s+1)} ℏ
Spin multiplicity (S) = 2s + 1
Where, s = total spin of the electrons.
Quantum numbers, atomic orbitals and electronic configuration
Lesson explainer: Quantum number chemistry
Atomic orbitals and quantum numbers
Quantum mechanical model of an atom |ChemFam #09|
A case study about the growth mechanism of CNT |ChemFam #08|
Carbon Nanotubes (Buckytubes): Types and Synthesis |ChemFam #07|
Nanomaterials: Classification and Approach for Synthesis |ChemFam #06|
Azadirachtin: Isolation, Extraction and Mechanism of Action |ChemFam #05|
Woodward-Fieser Rules for Calculating λmax |ChemFam #04|
Chemistry in ancient India |ChemFam #03|
How do soaps clean the dirt? |ChemFam #02|
What is anti egg white injury factor? |ChemFam #01|
PS The thumbnail image is being created by me using canva.com

