Ultraviolet and visible spectroscopy primarily deals with the recording of the absorption of radiations in the ultraviolet(UV) and visible regions of the electromagnetic spectrum. The UV region extends from 10 to 400 nm. It is further subdivided into the near ultraviolet region (200-400 nm) and the far or vacuum ultraviolet region (10-200 nm). The visible region extends notably from 400 to 800 nm.
Organic chemists use UV and visible spectroscopy mainly for detecting the presence and also to elucidate the nature of the conjugated multiple bonds or aromatic rings.
At a given wavelength the molar absorptivity of an organic compound is constant. The intensity of an absorption band in the UV or visible spectrum is usually expressed as the molar absorptivity at maximum absorption, εmax or log10εmax· The wavelength of the maximum absorption is denoted by λmax
Woodward formulated a set of empirical rules for calculating λmax or predicting in conjugated acyclic and six-membered ring dienes. These rules, modified by Fieser and Scott on the basis of wide experience with dienes and trienes, are called Woodward-Fieser rules and are summarized in a table below. First, we discuss the following terms used in Woodward-Fieser rules.
Woodward-Fieser Rules for Calculating λmax in Conjugated Doenes and Trienes
| Conjugated Alkenes | Base Value |
|---|---|
| Homoannular Diene | 253 nm |
| Heteroannular Diene | 215 nm |
| Butadiene or Cyclic Conjugated Diene | 217 nm |
| Increment for Each Alkyl Substituent | 5 nm |
| Increment for Each Exocyclic Double Bond | 5 nm |
| Increment for Each Double Bond Extending Conjugation | 30 nm |
Homoannular Diene
In homoannular dienes, conjugated double bonds are present in the same ring and having s-cis (cisoid) configuration (s =single bond joining the two doubly bonded carbon atoms)

Heteroannular Diene
On the contrary in heteroannular dienes, conjugated double bonds are not present in the same ring and these have s-trans (transoid) configurations:

Exocyclic Conjugated Double Bonds
The carbon-carbon double bonds that projects just outside a ring are called exocyclic double bonds. For example
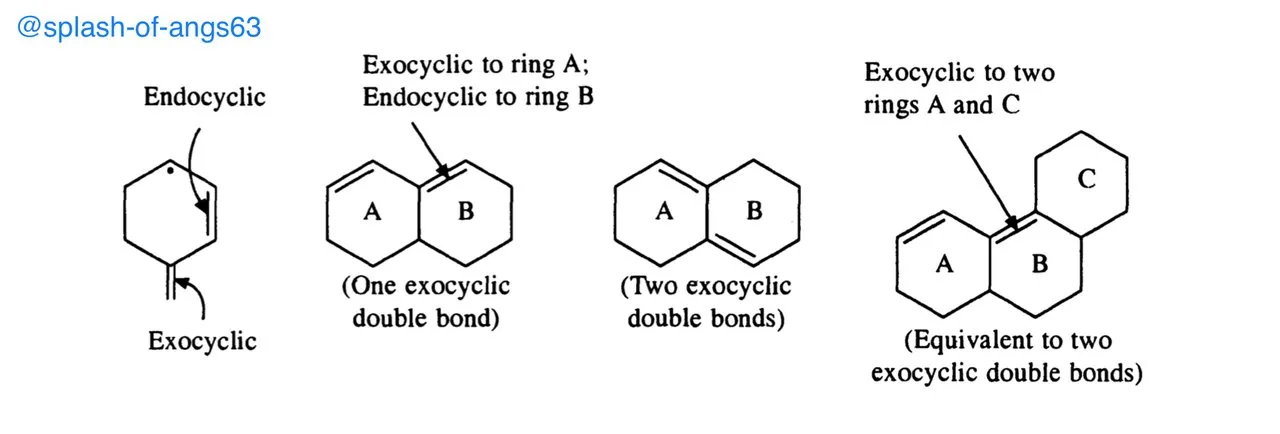
Note that the same double bond may be exocyclic to one ring, while endocyclic to the other and sometimes the same double bond may be exocyclic to two rings simultaneously.
Alkyl Substituents and Ring Residues


In compounds containing both homoanular and heteroannular diene systems, the calculations are based on the Ionger wavelength (253 nm), i.e. the homoannular diene system.
Example 1
For the following organic compound we can confirm that the parent system is a heteroannular diene. So the base value is 215 nm. Now the compound has three alkyl substituents attached to the parent simple butadiene system. Since each alkyl substituent contributes 5 nm to the base value, So these three alkyl side chains will contribute total of 15 nm. Again, For the two cyclohexene rings, one ring has an exocyclic double bond attached to it. Hence, it will contribute 5 nm to the base value. To conclude, the λmax is obtained by adding all these values which came to about 235 nm.Example 2
Similarly in this compound the parent system is a heteroannular diene. So the base value is again 215 nm. Now the 4 alkyl substituents contribute total of (4x5)=20 nm. By adding this value to the base value, we obtained λmax = 235 nm

Example 3
For the compound 3 the parent compound is a homoannular diene. So the base value is 253 nm. We can find 5 alkyl side chains attached to the conjugated dienes. 1 exocyclic double bond contributes 5nm. Moreover it has 2 double bonds extending conjugation on the parent homoannular diene. It contributes 60 nm to the base value. So the λmax was found to be 343 nm after adding all the values.
Example 4
For the compound 4, the parent system is a simple butadiene. Hence the base value is 217 nm. The 4 alkyl side chains on the parent system contributes 20 nm. Contribution of one exocyclic double bond is 5 nm. Therefore λmax = 242 nm

Woodward-Fieser Rules for Calculating λmax in α,β-Unsaturated Carbonyl Compounds
Compounds containing a carbonyl group (C=0) in conjugation with an ethylenic groups (C=C) are called enones. Similar to dienes and trienes, there are set rules called Woodward-Fieser rules for calculating or predicting λmax in α,β-unsaturated carbonyl compounds (enones). These rules first framed by Woodward and modified by Fieser and Scott are given below
| α,β-unsaturated carbonyl compounds | Base Value |
|---|---|
| Either Acyclic or 6 membered Cyclic α,β-unsaturated carbonyl compounds | 215 nm |
| 5 membered cylic α,β-unsaturated carbonyl compounds | 202 nm |
| Increment for Each Exocyclic Double Bond | 5 nm |
| Increment for Each Diuble Bond Extending Conjugation | 5nm |
| Increment for Each Alkyl Substituent at α-Position | 10 nm |
| Increment for Each Alkyl Substituent at β-Position | 12 nm |
| Increment for Each Alkyl Substituent at γ or γ+ Position | 18nm |
| Increment for Each Homoannuler Conjugated Diene | 39 nm |
Example 5
For example 5 the parent system is a 6 membered cyclic α,β-unsaturated carbonyl compounds. The base value corresponding to it is 215 nm. There are two side chains at position β to the carbonyl carbon, which contributes 24 nm. There is also an exocyclic double bond that contributes 5 nm as well. Hence, λmax was found to be 244 nm after adding all the values.
Example 6
For this, the parent system is a 5 membered cyclic α,β-unsaturated carbonyl compound. So the base value is 202 nm. 1 β(beta) alkyl side chain contributes 12 nm. 1 γ(gamma) alkyl side chain contributes 18 nm and 1 δ(delta) alkyl side chain with respect to the carbonyl carbon contributes 18 nm as well. 1 double bond extending conjugation from the parent system contributes 30 nm. Hence, after adding all these values λmax was found to be 285 nm.

Example 7
For this compound the parent system is a 6 membered cyclic α,β-unsaturated carbonyl compound. So the base value is 215 nm. 1 α alkyl side chain with respect to the carbonyl carbon contributes 10 nm. One γ alkyl side chain contribues 18 nm. 1 exocyclic double bond contributes 5 nm and one double bond extending conjugation from the parent system contributes 30 nm. There is a one homoannuler diene also which contributes 39 nm to the base value. So, the final value of λmax is 317 nmExample 8
Here, the parent system is a 6 membered cyclic α,β-unsaturated carbonyl compound. So, the base value is 215 nm. 1 side chain at β position to the carbonyl carbon contributes 12 nm. There is 3 alkyl side chains at position γ or more. So each of them contributes 18 nm. There are two double bonds extending conjugation from the parent system. So, these two contributes 30 nm each. The two exocyclic double bonds also contributes 5 nm each to the base value. Hence, the λmax was found to be 349 nm for the said compound.

Organic Chemistry by Jonathan Clayden, Nick Greeves and Stuart Warren
Organic Spectroscopy By William Kemp
Elementary Organic Spectroscopy By Y.R. Sharma
Chemistry in ancient India |ChemFam #03|
How do soaps clean the dirt? |ChemFam #02|
What is anti egg white injury factor? |ChemFam #01|
PS : All the images are drawn by me through KingDraw app and my iPad


