Greetings to everyone! In my previous post, we were discussing about membrane separations in natural gas system. When we talk about natural gas purification or natural gas separation, we have to take out the various types of impurities present and water is the main impurities in natural gas and for that we need to know the water content in the natural gas so that we can design how to dehydrate the water. So, today we will be discussing about the estimation of water content in natural gas.
WHAT WE SHALL LEARN?
We shall be learning about the significance of water content in natural gas, how to specify the water content, factors affecting water content and most importantly the various estimation method of water content.
SIGNIFICANCE OF WATER CONTENT IN NATURAL GAS
First, let us have a look at the significance of water content in natural gas. Water has some bad effects or acts as impurity in the separation or purification of natural gas.
- Water in natural gas degrades the fuel value of natural gas i.e., the natural gas with lots of water will be having less calorific value. So, we shall not be able to derive much thermal energy by burning this kind of natural gas with high amount of water.
- Water also dictates the formation of acids (with sour gases) and hydrates (with methane, ethane, nitrogen, carbon dioxide etc). Carbon dioxide and Hydrogen sulphide present in water content may react with water to form weak acids which may corrode the pipelines and the equipment. The hydrates formed by the water content are solid particles and they may get deposited inside the pipelines or in the equipment and heat exchangers.
- The hydrates formed will either generate pressure drop, whereby impeding the flow of the gas and reducing the efficiency of heat transfer. That is how it will affect the dehydration system.
SPECIFICATION OF WATER CONTENT
There are various ways of specifying the water content.
☑ water content can be specified in terms of mass of water per unit volume of gas (mg/Sm3 for gases.
☑ Another way is parts per million by volume (ppmv) for gases.
☑ Parts per million by mass (ppmw) for liquids.
It is interesting to note that ppmv and ppmw require molecular weight of hydrocarbon molecules for conversion to other concentrations.
☑ Lastly we also have the dew point temperature (DPT) because when depending on the amount of water in any gas, the gas will be having different dew points. So, dew point is an indication of the water content and this is used for both gases and liquids and generally there is no simple method to convert the dew point temperature i.e., DPT into ppmv or ppmw.
CONVERSION OF PPMV TO mg/m3
Now, we shall see how to do some kind of basic conversion among these terms.
☑ ppmv is same as ppm on molar basis.
1 ppmv = 10-6 × (kmol species i / kmol gas mixture)
= 10-6 × (Mi kg / kmol of gas mixture)
= Mi × (mg species i / kmol of gas mixture)
Where Mi is molecular weight of species i in kg/kmol.
☑ Applying ideal gas law,
V = n × RT / P m3 [R in kj/kmol K, T in K and P in kPa]
1 ppmv = MiP / RT × (mg species / m3 of gas mixture)
CONVERSION OF PPMW TO mg/m3
1 ppmw = 10-6 × (kg species i / kg liquid mixture)
= 1 / ρavg × (mg species i / m3 liquid mixture.
Where ρavg is in kg / m3
1 ppmw = 10-6 × (kg species i / kg gas mixture)
= Mavg × (mg species i / kmol gas mixture)
= PMavg / RT × ( mg species i / m3 of gas mixture.
FACTORS AFFECTING THE WATER CONTENT OF NATURAL GAS
Water content in the natural gas drops with an increase in molecular weight and salinity. It decreases with the decrease in temperature, pressure and amount of liquified acid gases (H2S or CO2) which significantly reduce the solubility of water in natural gas.
ESTIMATION OF WATER CONTENT IN NATURAL GAS
We have various charts and analytical method to estimate the water content in natural gas.
CHARTS
✓ Charts are used because they are simple and easier to estimate the water content than analytical methods.
✓ They also have some drawbacks due to which they are not so widely used. They do not find much use due to lack of accurate data and difficulty in interpolation.
There are various charts depending on the varieties of natural gas used to separate. Some of which are -
- McKetta-Wehe pressure-temperature correlation chart.
- Witchert water content ratio chart.
- Campbell chart.
- Robinson chart.
Here, we will see only about McKetta-Wehe pressure-temperature correlation chart.
McKetta-Wehe pressure-temperature correlation chart:
It is the most popular charts in estimating water content in natural gas system. It can estimate water content having methane content as low as 0.70 mole fraction. Gas gravity should never be used to account for the presence of H2S, CO2 and hydrocarbons. The hydrate line is approximate and should not be used to estimate gas hydrate content. The composition dependency of water content cannot be obtained.
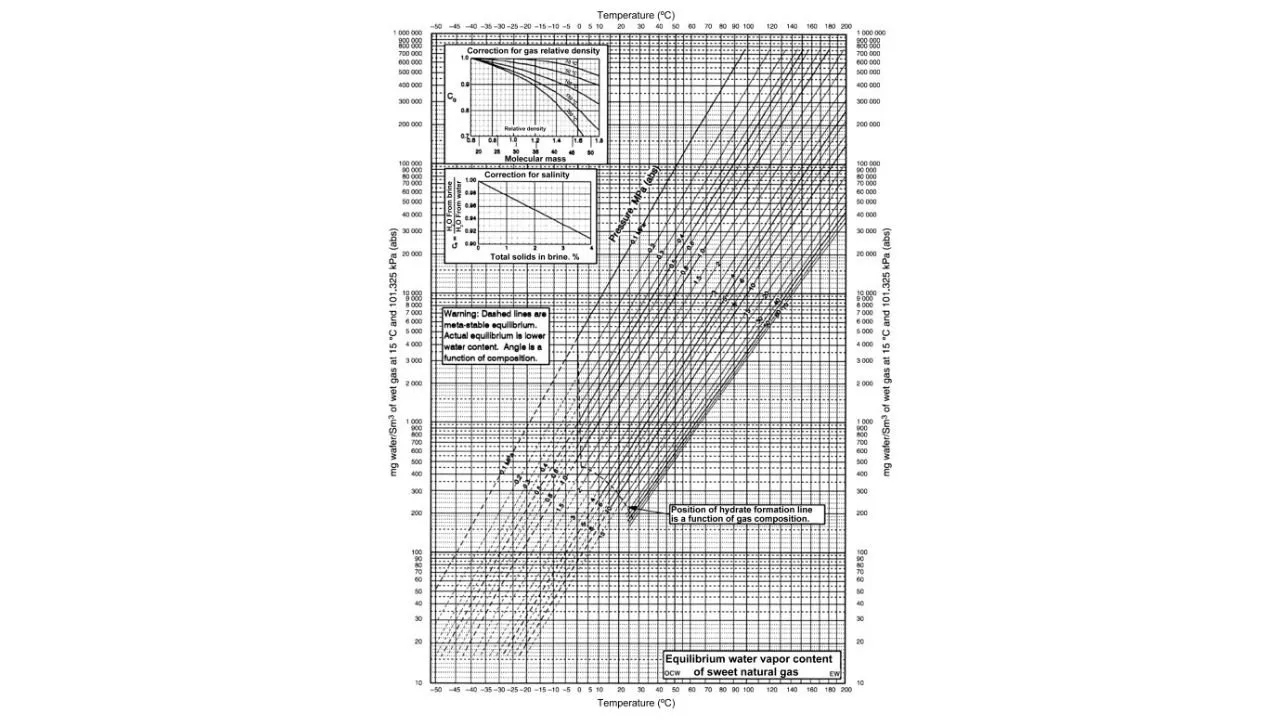
Here, we find that on the y-axis, we have the water content, on the x-axis, we have temperature and all these lines represent the various pressure. We also have some correction factors for salinity, for the gas relative density. We can also see a dotted line which represents the position where the hydrates may be formed. So, it is just a qualitative indication above which there could be hydrate formation.
ANALYTICAL METHODS
These methods are advantageous over the charts as they are more accurate, quick, convenient and easy to program. The types of analytical methods includes -
Simplified thermodynamic models and Empirical/Semi-empirical correlations.
Thermodyanic model: They are accurate and applicable to liquid-vapor, hydrate-vapor, ice-vapor and liquid-hydrate-vapor regions. In this models, the basis is that at vapor-liquid equilibrium, the fugacity of a component is same in both vapor and liquid phases.
Empirical or semi-empirical correlations: These are obtained by regression of experimental data. They are simple, convenient and accurate within specified conditions. But they are less accurate in the presence of heavy hydrocarbons.
Analytical methods for sour natural gas:
The correlations incorporate acid gas effect on the physical properties. They are based on the calculation of phase equilibrium in water-hydrocarbon-acid gas system. They do not require the estimation of the water content on sweet natural gas in advance.
Maddox’s component contribution model:
It assumes water content in sour natural gas as the sum of the contributions by sweet natural gad, hydrogen disulphide and carbon dioxide. The water content is given by,
WH2O,sour = WHC,sweet + YCO2WCO2 + YH2S
Where subscripts ‘HC’ and ‘sour’ refers to hydrocarbons and sour natural gas respectively. All WH2O in mg/Sm3, pressure in MPa and temperature in K.
Also,
log WNHC = ao + a1 log P + a2(log P)2
Where NHC stands for non-hydrocarbons such as H2S and CO2. The coefficients ao, a1 and a2 can be obtained by the table shown below.

Formula calculation methods of water content in sweet natural gas and their adaptability analysis
Estimation of Water Content in Sour Gases
Advances in Estimating Water Content of Natural Gases
Membrane Separation in Natural Gas System |ChemFam #16|
Design of distillation column |ChemFam #15|
Separation Technique: Distillation |ChemFam #14|
Transmission Electron Microscope: Principle and Working |ChemFam #13|
Scanning Electron Microscope: Principle and Working |ChemFam #12|
Drugs: Classification and drug-target interaction |ChemFam #11|
What are orbitals and quantum numbers? |ChemFam #10|
Quantum mechanical model of an atom |ChemFam #09|
A case study about the growth mechanism of CNT |ChemFam #08|
Carbon Nanotubes (Buckytubes): Types and Synthesis |ChemFam #07|
Nanomaterials: Classification and Approach for Synthesis |ChemFam #06|
Azadirachtin: Isolation, Extraction and Mechanism of Action |ChemFam #05|
Woodward-Fieser Rules for Calculating λmax |ChemFam #04|
Chemistry in ancient India |ChemFam #03|
How do soaps clean the dirt? |ChemFam #02|
What is anti egg white injury factor? |ChemFam #01|
PS The thumbnail image is being created by me using canva.com using template image from EH

