
The COVID-19 Pandemic would never have happened without the PCR test. In 2020, the year of the pandemic, most of us probably did not know anyone who died from the disease. I personally did not even know anyone who contracted covid and recovered. My first experience of this supposedly novel disease was in January 2021, when my 86-year-old mother and a dozen other residents of her care home tested positive for the disease one week after receiving their first dose of the Pfizer vaccine. Later, in the winter of 2021-22, several other members of my immediate family tested positive for covid—in each case a few months after receiving their second dose of the vaccine. In every one of these cases, the diagnosis was made entirely on the basis of a PCR test.
Without this test, most cases of covid either would not be diagnosed as any disease—“asymptomatic”—or would be diagnosed as one of the many endemic respiratory illnesses with similar or identical symptoms, such as the common cold, influenza, pneumonia, bronchitis, or COPD. It is alleged, however, that a positive PCR test proves that the patient is infected with a specific pathogenic virus, SARS CoV-2, which causes a specific disease, COVID-19.
PCR has revolutionized the science and practice of epidemiology. Even Kary Mullis, the inventor of PCR, did not see anything inherently wrong with the use of this technique as a diagnostic tool. It was, in fact, one of the many applications which he envisaged PCR might be put to:
Years later I invented the polymerase chain reaction. I was a professional scientist, and I knew what I had discovered. It was not the speculations of a kid about the universe and time reversal. It was a chemical procedure that would make the structures of the molecules of our genes as easy to see as billboards in the desert and as easy to manipulate as Tinkertoys.
PCR would not require expensive equipment, and it would find tiny fragments of DNA and multiply them billions of times. And it would do it quickly.
The procedure would be valuable in diagnosing genetic diseases by looking into a person’s genes. It would find infectious diseases by detecting the genes of pathogens that were difficult or impossible to culture. PCR would solve murders from DNA samples in trace materials—semen, blood, hair. The field of molecular paleobiology would blossom because of PCR. Its practitioners would inquire into the specifics of evolution from the DNA in ancient specimens. The branchings and migrations of early man would be revealed from fossil DNA and its descendant DNA in modern humans. And when DNA was finally found on other planets, it would be PCR that would tell us whether we had been there before or whether life on other planets was unrelated to us and had its own separate roots. (Mullis 104-105)
Kary Mullis was actually working as a consultant in this very field when he first became interested in the true cause of AIDS:
Four years later I was working as a consultant at Specialty Labs in Santa Monica. Specialty was trying to develop a means of using PCR to detect retroviruses in the thousands of blood donations received per day by the Red Cross. I was writing a report on our progress for the project sponsor, and I began by stating, “HIV is the probable cause of AIDS.” (Mullis 171)
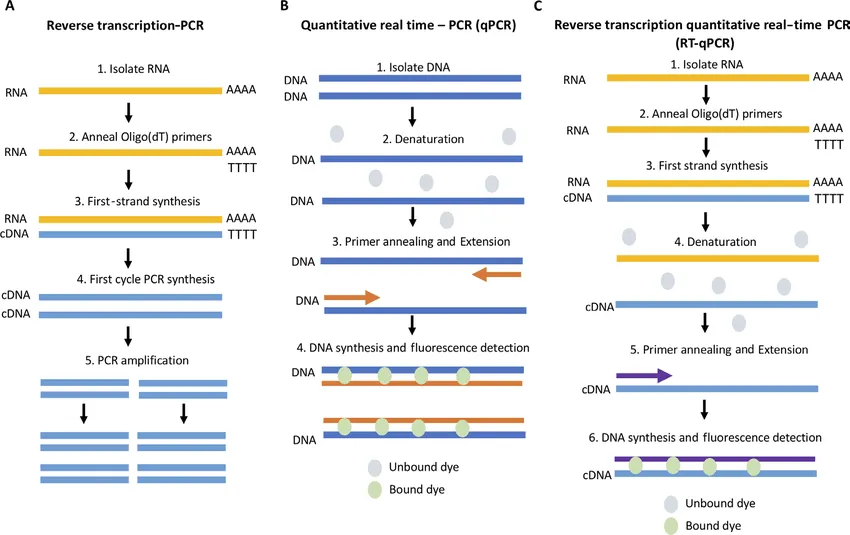
SARS-CoV-2 is, allegedly, a retrovirus—that is, a virus whose genetic material is in the form of RNA rather than DNA. A variant of PCR known as RT-PCR is used to detect and polymerize target segments of RNA. The Reverse Transcription Polymerase Chain Reaction first converts the RNA strands in the sample into complementary strands of DNA—known as cDNA—using an enzyme called Reverse Transcriptase. Once this is done, the standard PCR procedure is then applied to the cDNA. PCR can also be used—allegedly—to estimate the quantity of the target DNA in the sample. This variant of the test is called qPCR, or quantitative Polymerase Chain Reaction. Similarly, there is a corresponding variant of RT-PCR called RT-qPCR.

In order to use RT-qPCR to detect a particular retrovirus, several things must first be in place:
- The retrovirus must be discovered and isolated.
- The genome of the retrovirus must be sequenced.
- A gene unique to that retrovirus must be identified in this genome.
A unique gene—or, rather, its cDNA equivalent—can then be used as the target sequence for the PCR test to detect and amplify. The two primers used in the PCR are chosen to bookend the gene’s cDNA in the manner described in the last article, so that the PCR will exponentially amplify only the target gene, assuming that it is present.
Whether these three initial steps have actually been taken in the case of SARS CoV-2 and COVD-19 will be the subject of later articles. In this article I want to investigate the actual procedure of PCR and see whether there are any practical problems or difficulties that undermine its use as a diagnostic tool.
Diagnosis
The problem with the diagnostic use of RT-PCR is that it cannot possibly tell you whether someone is ill, or whether they are infectious, or whether they are likely to become ill or infectious. Even if we accept all the necessary assumptions—that SARS CoV-2 exists, that it causes COVID-19, that a positive PCR test means that it is present in the sample—we cannot draw any further conclusions other than that the test replicated the target gene to the point where it could be detected by the chemist carrying out the test.
There are many ways in which RT-PCR may be misused or abused. One of these involves setting the cycle threshold (Ct) too high. The cycle threshold is the number of times the polymerase chain reaction is allowed to occur. In theory, each cycle doubles the number of samples of the target gene. When the cycle threshold has been reached, the target gene either has been amplified sufficiently to be detected (a positive test) or has not been amplified sufficiently to be detected (a negative test). Throughout the COVID-19 Pandemic, there was never an internationally recognized Ct that ought to be applied. In fact, in most cases the cycle threshold was not even mentioned in the results of a PCR test. And opinions on this subject differed widely.
In 1997, long before the pandemic, Kary Mullis identified this as a possible Achilles heel of the PCR test:
With the PCR if you do it well, you can find almost anything in anybody. It starts making you believe in the sort of Buddhist notion that everything is contained in everything else, right? I mean because if you can amplify one single molecule up to something that you can really measure, which PCR can do, then there’s just very few molecules that you don’t have at least one single one of ’em in your body. Okay? So that can be thought of as a misuse of it, just to claim that it’s meaningful. (Kary Mullis, Timestamp = 48:37)

Mullis spent much of his later life challenging presumptions about the use of the PCR as a medical diagnostic tool ... Mullis asserted many times that the PCR is not a diagnostic tool, it is a research and analytical tool. It cannot diagnose an infection. (Coppolino 19)

In an interview with Celia Farber, the Canadian researcher David Crowe expressed the problem thus:
PCR is really a manufacturing technique,” Crowe explained. “You start with one molecule. You start with a small amount of DNA and on each cycle the amount doubles, which doesn’t sound like that much, but if you, if you double 30 times, you get approximately a billion times more material than you started with. So as a manufacturing technique, it’s great. What they do is they attach a fluorescent molecule to the RNA as they produce it. You shine a light at one wavelength, and you get a response, you get light sent back at a different wavelength. So, they measure the amount of light that comes back and that’s their surrogate for how much DNA there is. I’m using the word DNA. There’s a step in RT- PCR test which is where you convert the RNA to DNA. So, the PCR test is actually not using the viral RNA. It’s using DNA, but it’s like the complimentary RNA. So logically it’s the same thing, but it can be confusing. Like why am I suddenly talking about DNA? Basically, there’s a certain number of cycles.”
This is where it gets wild.
“In one paper,” Crowe says, “I found 37 cycles. If you didn’t get enough fluorescence by 37 cycles, you are considered negative. In another, paper, the cutoff was 36. Thirty-seven to 40 were considered “indeterminate.” And if you got in that range, then you did more testing. I’ve only seen two papers that described what the limit was. So, it’s quite possible that different hospitals, different States, Canada versus the US, Italy versus France are all using different cutoff sensitivity standards of the Covid test. So, if you cut off at 20, everybody would be negative. If you cut off a 50, you might have everybody positive.” (Celia Farber)
Question: How many cycles are necessary for a single molecule to be amplified to the point where it can be detected by fluorescence (ie to the point where the PCR test is positive)?
Answer: 35.
For most instruments, once you get above a cycle of about 35—of all instruments, really—then you start worrying about the reliability of your result, because that would be roughly equivalent to a single copy. So you would hope, what you want to do is you want to be certain that—or you want to try and be sure that—the results you get are in the 20-30s. (The Infectious Myth 251, Timestamp = 27:00)

In July 2020, Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases, expressed a similar opinion on the netcast This Week in Virology:
If you get a [positive result using a] cycle threshold of 35 or more, then the chances of it being replication competent are minuscule. (This Week in Virology, Timestamp = 4:17)
Replication competent means that the virus particles are capable of infecting cells and replicating to produce additional infectious particles.
But the cycle threshold is just one way in which the PCR test might be misused. There are many more that must be investigated before we even come to consider what would be an appropriate Ct. These fall into two broad categories:
Problems with the science of virology itself.
Problems with the application of the PCR test and the interpretation of its results.
The first category will be the subject of later articles. In the remainder of this article, we will investigate problems that fall into the second category.

Theory and Practice
In theory, qPCR and RT-qPCR sound like godsends for the medical practice. A laboratory test that can identify the presence of a specific pathogen which is too small to image in a light microscope: that is surely revolutionary. But there are actually huge practical and logistical problems involved in carrying out a PCR test. If these are not understood and addressed, the results are open to misinterpretation. Kary Mullis himself was probably the first to discover that the theory and practice of PCR were two very different beasts:
In the early years of PCR, no one could figure out why certain methods of doing it turned out to be better than others. As I first envisioned PCR, each cycle would cause the amount of target DNA to double. The first cycle would provide twice as much, the second cycle four times as much, the third cycle eight times. But often by the tenth cycle, it would not completely double. It would increase by a factor of 1.8, then 1.5, and 1.3. Something was running out or something was being made that interfered with the process. It meant that calculations based on a consistent doubling could be way off. Like compound interest with a variable rate.
In PCR there are about twenty different things you have to measure out, each of them dependent on all the others. In figuring out how to perform the reaction so that each cycle would result in a complete doubling of the target molecule, a major problem I encountered was that I had to deal with several completely different systems for measuring the amounts of things. Some of the molecular ingredients were measured in grams, some of them in esoteric units of activity, and some of them by how much ultraviolet light they would absorb. The reasons for all this were historical and constituted a real pain in the science of chemistry that no one had taken the trouble to fix ...
I created an entirely new system for comparing substances, the details of which would be of considerable interest to only a small group of people. But it was by using this system that I was finally able to figure out why people doing PCR were getting inconsistent results. When I compared the number of molecules in each of my ingredients, it became obvious that in many cases there simply weren’t enough enzyme molecules to react with the DNA molecules that had to be processed. Nobody had realized that the limiting factor in doing PCR is simply the number of molecules of the enzyme, because they had no way of knowing how many molecules of enzyme they were using.
When I started experimenting with PCR, I never knew how many molecules of enzyme I had in my solution. Enzyme amounts are expressed in units of activity, how many molecules of something it will turn into something else, under a standard set of conditions per unit of time. Only after I converted each of the ingredients needed to do PCR to a simple system of counting molecules did the problem, and the solution, become obvious. Keep the number of things the enzyme is going to interact with smaller than the number of molecules of the enzyme. Simplicity is embarrassing when you have to work for months to achieve it. (Mullis 96 ... 99-100)

Long before COVID-19, the Centers for Disease Control and Prevention (CDC) had been aware of the shortcomings of PCR. In 2007, an article was published by the CDC’s Morbidity and Mortality Weekly Report with the title Outbreaks of Respiratory Illness Mistakenly Attributed to Pertussis—New Hampshire, Massachusetts, and Tennessee, 2004-2006:
This report describes two hospital outbreaks and one community outbreak of respiratory illness during 2004--2006 in New Hampshire, Massachusetts, and Tennessee that were attributed initially to pertussis. However, subsequent investigations revealed negative or equivocal laboratory results and epidemiologic and clinical features atypical of pertussis, suggesting that pertussis was not the cause of these outbreaks ...
Presumed false-positive PCR test results in persons with nonspecific clinical features, such as rhinorrhea, sneezing, and sore throat, have raised concerns regarding the widespread application of PCR in an outbreak setting. No standardized PCR protocols for pertussis testing exist; approximately 100 different assays that use the IS481 target sequence have been documented. Laboratories vary in DNA purification techniques, primers and probes used in testing, and quality assurance procedures. Although these assays might undergo analytic sensitivity testing for technical performance standards (e.g., detection limits and reproducibility), a limited number of laboratories have established the accuracy of their PCR test. In addition, as illustrated in the Massachusetts outbreak, interpretation of PCR results can vary among laboratories.
The outbreaks described in this report illustrate the limitations of relying solely on PCR assays to confirm pertussis. PCR is an important tool for diagnosing individual cases of pertussis in persons for whom a high index of suspicion exists and for whom timely treatment and PEP are essential. However, the positive predictive value can be lower if PCR is used as a screening tool without culture confirmation during a suspected pertussis outbreak. Overreliance on the results of PCR assays can lead to implementation of unnecessary and resource-intensive control measures (e.g., case identification, antimicrobial treatment, furlough of ill persons, and administration of PEP). In outbreak settings, positive PCR results should be interpreted in conjunction with epidemiologic investigation, evaluation of clinical symptoms, and confirmation by culture. CDC recommends timely collection and testing (early in the course of illness and during the initial stages of the outbreak) of nasopharyngeal specimens for culture in at least a subset of persons who are symptomatic to confirm pertussis as the etiology of the outbreak. Absent or inconsistent supporting data and negative pertussis cultures in appropriately collected specimens should prompt testing for alternate pathogens. (Morbidity and Mortality Weekly Report 24 August 2007)

Even the unimpeachable bastion of mainstream opinion, The New York Times reported on these shortcomings, using language that would be labelled today as dangerous medical misinformation:
There are no national data on pseudo-epidemics caused by an overreliance on such molecular tests, said Dr. Trish M. Perl, an epidemiologist at Johns Hopkins and past president of the Society of Health Care Epidemiologists of America. But, she said, pseudo-epidemics happen all the time. (The New York Times 22 January 2007)
Did a pseudo-epidemic occur in 2020?
And did matters improve over the course of the following thirteen years? Johns Hopkins’ own record of confirmed COVID tests and confirmed COVID deaths would suggest that the answer is a resounding No:
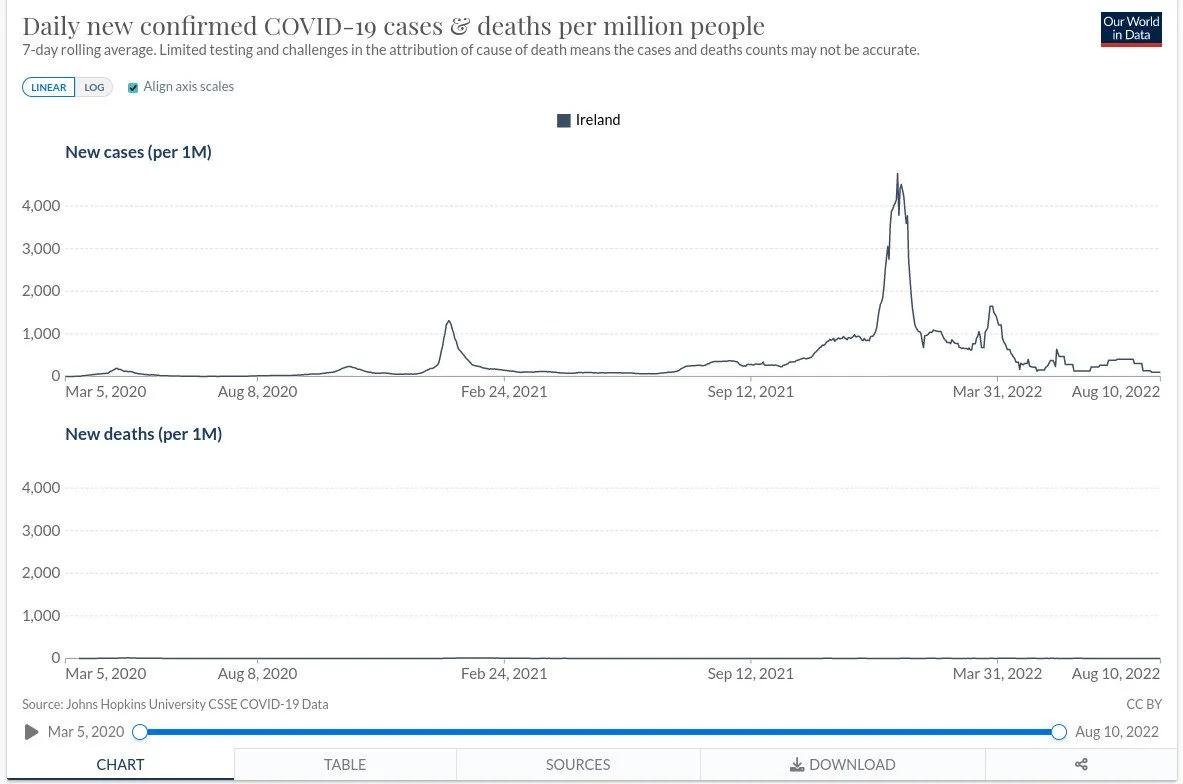
When the Johns Hopkins’ data for Ireland—confirmed cases and confirmed deaths—are displayed on identical axes, there is a gross disparity between the two. And this is typical of other countries. If we rescale the mortality graph to display some detail, and switch to logarithmic scales, there is certainly a correlation between the two:

But the huge disparity in numbers means that either the alleged pathogen is not very dangerous or there is a significant “false positive” problem—or both.
In 2009, Stephen Bustin, one of the world’s leading experts on the science and practice of RT-qPCR, raised concerns about the lack of consistency in the use of PCR:
Currently, a lack of consensus exists on how best to perform and interpret quantitative real-time PCR (qPCR) experiments. The problem is exacerbated by a lack of sufficient experimental detail in many publications, which impedes a reader's ability to evaluate critically the quality of the results presented or to repeat the experiments. (Bustin et al 2009:611)
Eight years later, the situation had not improved:
Poorly executed and inadequately reported molecular measurement methods are amongst the causes underlying the lack of reproducibility of much biomedical research. Although several high impact factor journals have acknowledged their past failure to scrutinize adequately the technical soundness of manuscripts, there is a perplexing reluctance to implement basic corrective measures. The reverse transcription real-time quantitative PCR (RT-qPCR) is probably the most straightforward measurement technique available for RNA quantification and is widely used in research, diagnostic, forensic and biotechnology applications. Despite the impact of the minimum information for the publication of quantitative PCR experiments (MIQE) guidelines, which aim to improve the robustness and the transparency of reporting of RT-qPCR data, we demonstrate that elementary protocol errors, inappropriate data analysis and inadequate reporting continue to be rife and conclude that the majority of published RT-qPCR data are likely to represent technical noise. (Bustin et al 2017:756)

Another anomaly of the PCR test involves the choice of primers. As we noted above, the primers are chosen to target a specific gene—allegedly a gene that is unique to SARS CoV-2. But sometimes a PCR test is carried out on a sample in which two different genes are targeted. If PCR is really doing what it is supposed to be doing, such a case should either detect both genes or fail to detect both genes. How could the test be positive for one gene and negative for the other? If both genes are unique to SARS CoV-2, then either they are both present in the sample and will be detected or they are both absent and will not be detected. If one is detected and not the other, this can only mean that PCR is not actually doing what it is said to be doing, or one of the tests is a false positive, or one of the test is a false negative. But, once again, there is no consensus on how to deal with such results. In some cases, the test is considered negative unless both genes are detected. In other cases, the test is considered positive if either gene is detected. In tests in which three genes are targeted, two out three detections is considered a positive test (David Crowe Podcast, Timestamp = 12:00).
The RT-qPCR involves numerous steps, and every one of those steps is subject to error (David Crowe Podcast, Timestamp = 13:50).
But let’s go to the very root of PCR and ask how precisely was SARS CoV-2 discovered.
And that’s a good place to stop.
References
- Stephen A Bustin et al, The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments, Clinical Chemistry, Volume 55, Issue 4, Pages 611-622 (2009)
- Stephen A Bustin et al, Talking the Talk, But Not Walking the Walk: RT-qPCR as a Paradigm for the Lack of Reproducibility in Molecular Research, European Journal of Clinical Investigations, Volume 47, Issue 10, Pages 756-774 (2017)
- Eric Francis Coppolino, The Covid Chronology, Version 5.1.2, Online (2022)
- Kary Banks Mullis, Dancing Naked in the Mind Field, Pantheon Books, New York (1998)
Image Credits
- Schematic Comparing RT-PCR, qPCR, and RT-qPCR: Grace Adams, A Beginners Guide to RT-PCR, qPCR and RT-qPCR, The Biochemist, Volume 42, Issue 3, Pages 48-53, Portland Press, London (2020)
- Kary Mullis: © Kent Clemenco (photographer), Fair Use
- Kary Mullis Quote: © Dona Mapston (photographer), Creative Commons License
- David Crowe: © Estate of David Richard Crowe, Fair Use
- A qPCR Laboratory: © Reaction Biology, Fair Use
- MMWR: Morbidity and Mortality Weekly Report, CDC, U.S. Department of Health & Human Services, Public Domain
- The New York Times: © The New York Times Company, Fair Use
- COVID-19 Confirmed Cases and Deaths (Ireland): © Johns Hopkins University & Medicine, Fair Use
- Covid Cases and Deaths Compared (Ireland): © Johns Hopkins University & Medicine, Fair Use
- Stephen Bustin: © Stephen Bustin (photographer), Fair Use
Video Credits
- Kary Mullis and Gary Null (1996): © Gary Null Productions, Fair Use
- This Week in Virology: © MicrobeTV, Inc, creative Commons License
Online Resources
- Our World in Data
- Johns Hopkins Coronavirus Resource Center
- Kary Mullis on PCR: Timestamp = 48:37
- RIP.ie
- EUROMOMO
- Rational Ground
