Greetings to everyone! In my previous post, we were discussing about the internal design of a distillation column. Now, we shall be learning about another type of separation used in the natural gas industries that is the Membrane Separation. This technique involves a process where a membrane is used to separate the components in a solution by rejecting undesirable substances and allowing the passes of desirable substances through the membrane.
WHAT SHALL WE LEARN?
In this particular post, we shall be learning about the application of membrane separation in natural gas system, different types of membrane operations, the applicability of membrane separation, properties of membrane and types of membranes in natural gas separation. We shall also be looking at mechanism of membrane gas separation and membrane modules.
APPLICATION OF MEMBRANE SEPARATION IN NATURAL GAS SYSTEM
We have some applications of membrane separation for the natural gas processing. Some of the major applications among them are the dehydration of natural gas, acid gas removal like CO2, H2S and the removal of nitrogen.
WHAT IS A MEMBRANE?
A membrane is a semi-permeable or we could call it as a permselective barrier that allows the selective permeation of certain species through it. The term permselective comes from two word- permeation and selective, that means what a membrane does that it allows some of the components to pass through it and does not allow some of the components to pass through it. That is how it is able to effect separation. So, this membrane separation is used both for liquid and gas separation. We are using the membrane separation at our household appliances like in the water purification. Microfiltration, ultrafiltration(UF), reverse osmosis(RO), dialysis etc which are used in water purifier. We shall be mostly looking at gas separation and not liquid separation in this particular post.
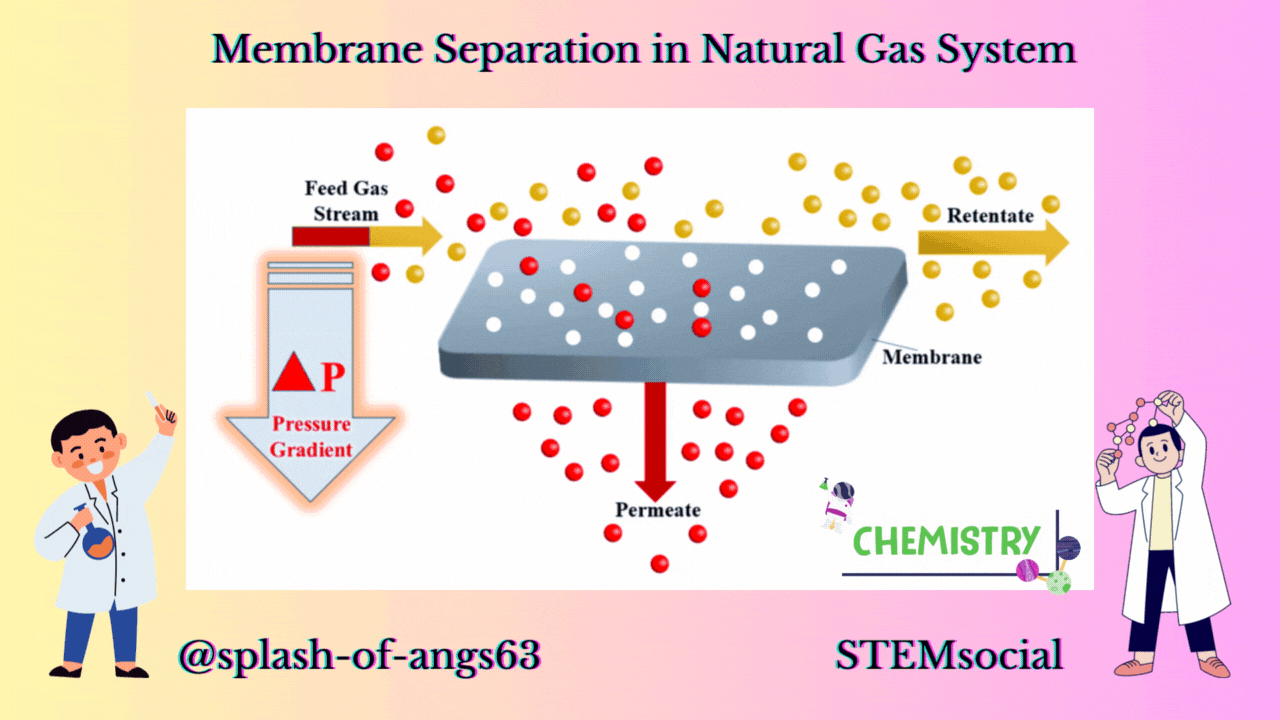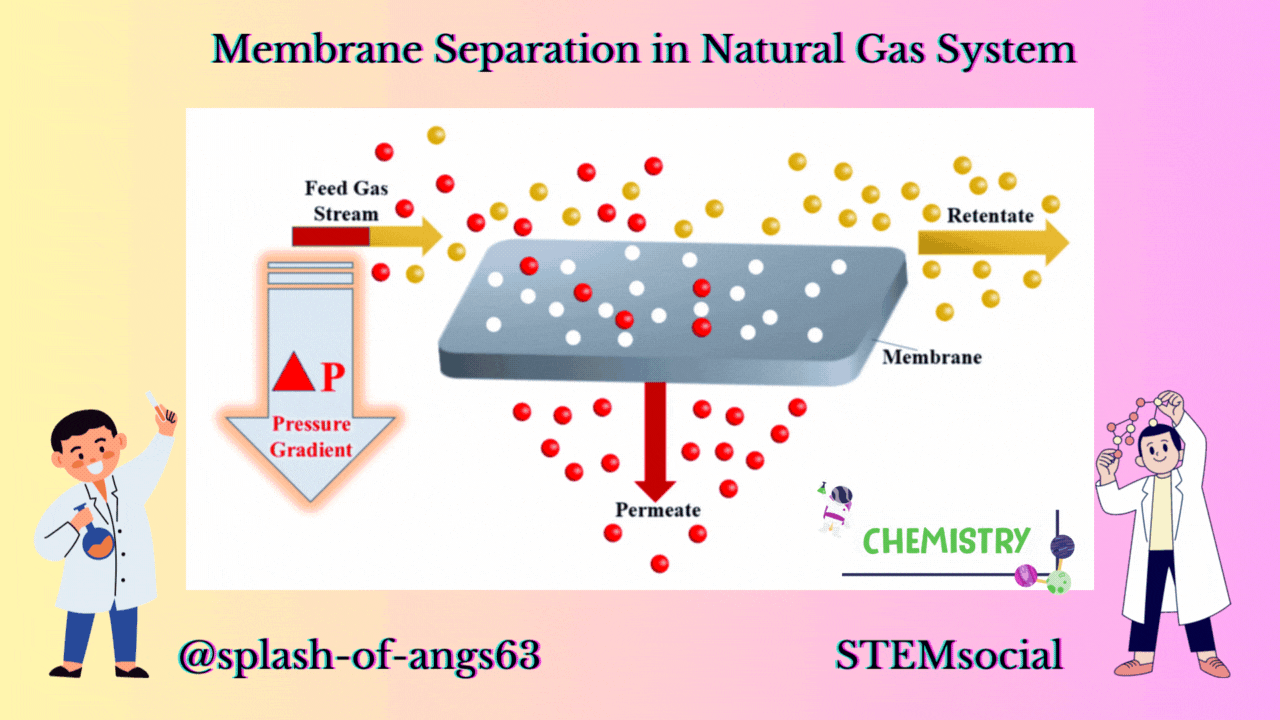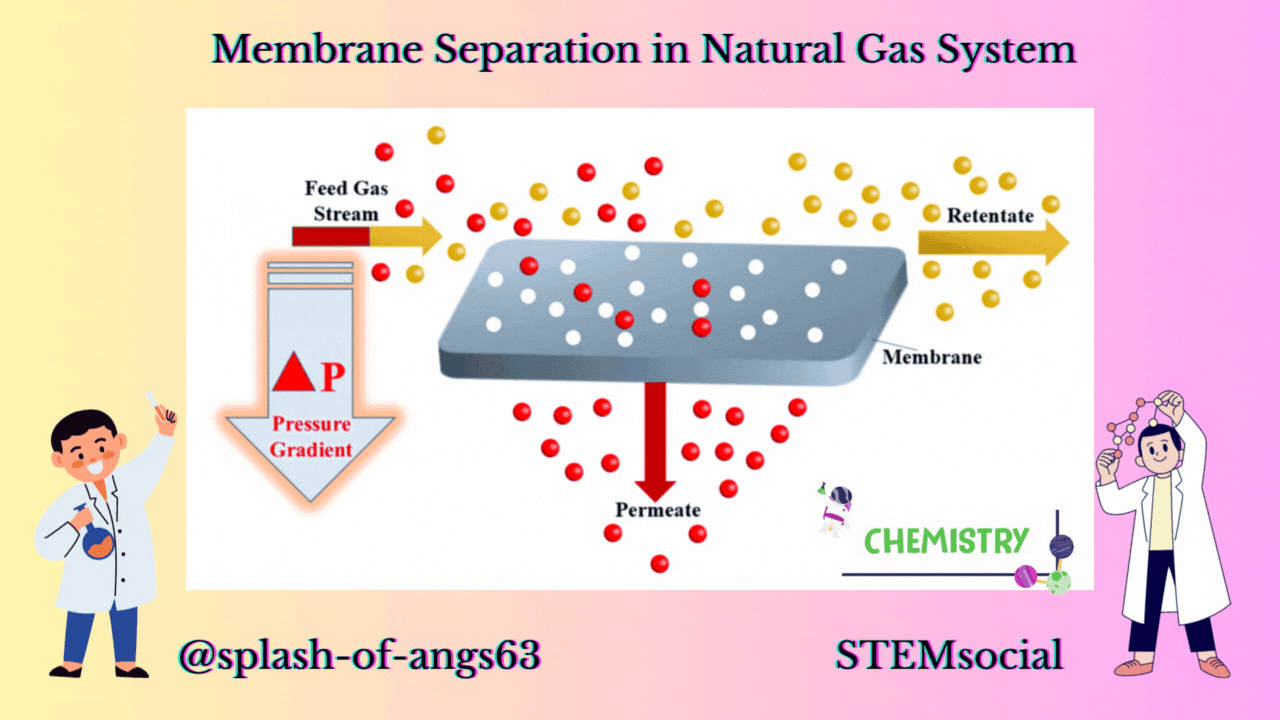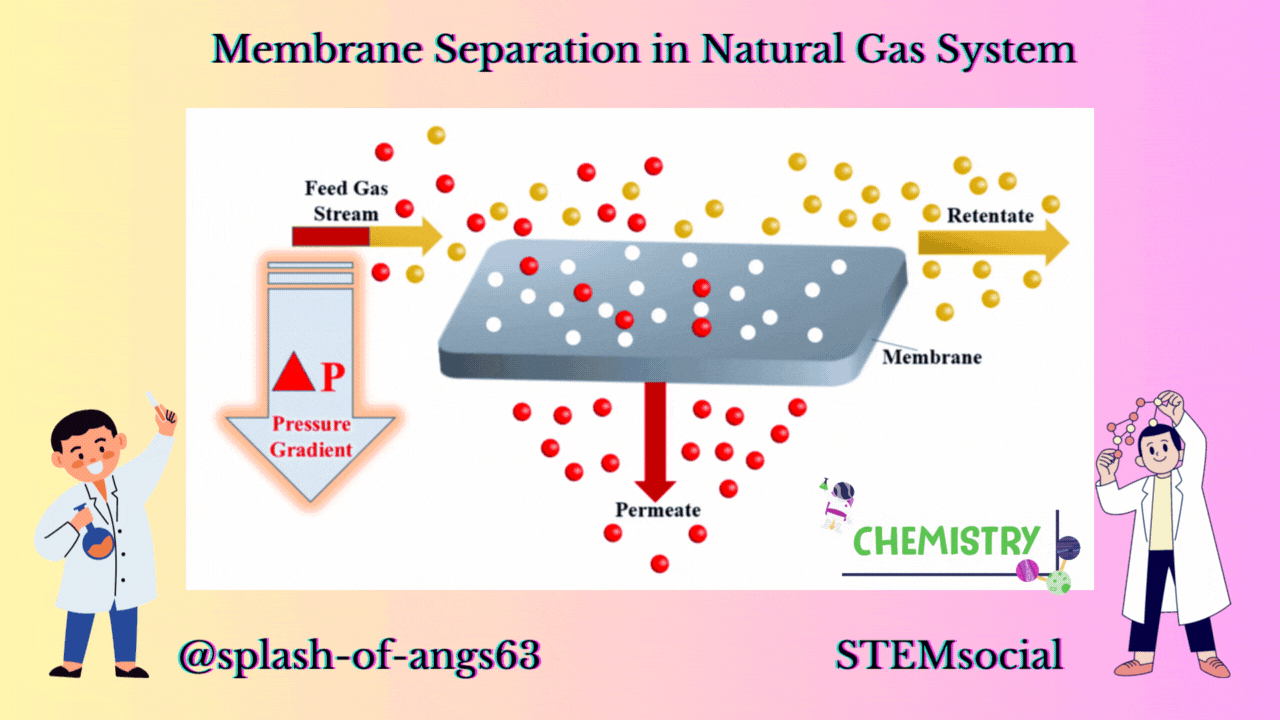
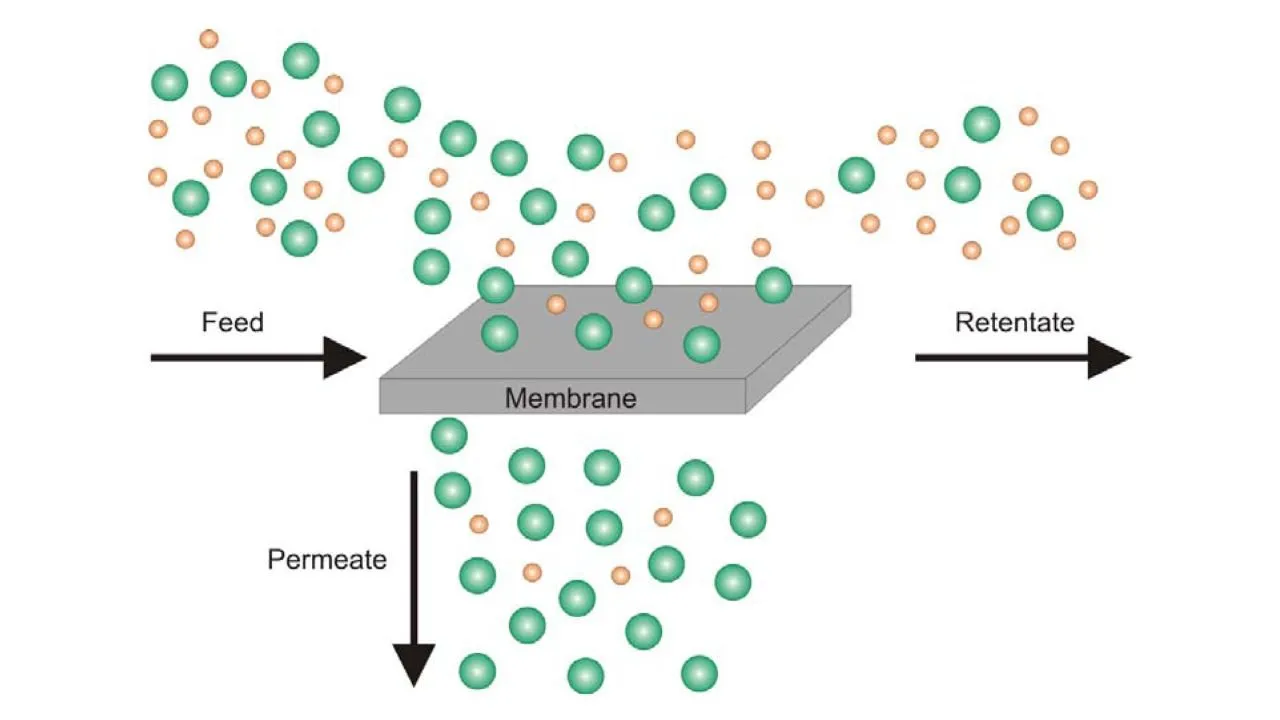
MEMBRANE CONFIGURATION
Now let us look at a typical membrane configuration. In the membrane configuration, we have three streams one is the feed which is separated into a retentate. A retentate is the total feed mixture that is retained by the membrane and another is the permeate and it is that part of the feed that goes through the membrane. The feed comes to the membrane and some of it passes through it as permeate and rest of the components passes over the membrane as retentate.
ADVANTAGES OF MEMBRANE SEPARATION
Let us have a look at the advantages this technique comes with- It is advantageous over other techniques such that it is light weight as it comes under modular construction. It also has large turndown ratio (A turndown ratio is a ratio of maximum to minimum capacity). Low maintenance and low operating cost are other advantages of this technique over other separation techniques.
MEMBRANE CLASSIFICATION
There are various ways of classifying the membrane. We have porous and non porous membranes. Porous membranes are used when the molecular size is more and non porous are used when the molecular sizes are comparatively less. The former is used for liquid separation and the latter is used for gas separation. As I have already mentioned we shall be looking at gas separation only so, non porous membranes are our point of interest.
A non-porous membrane have non-detectable pore at the limits of electron microscopy. So, this type of membranes are not totally non-porous.
DESIRABLE PROPERTIES OF MEMBRANE
There are some desirable properties of membranes which are worthy of pointing out-
☑ Good permeability
☑ High selectivity
☑ Defect free
☑ High stability whether mechanical, chemical or thermal
☑ Low fouling
☑ Reasonable useful life
☑ Ease of fabrication into compact, economical modules of high surface area per unit volume.
☑ Amenability to packaging
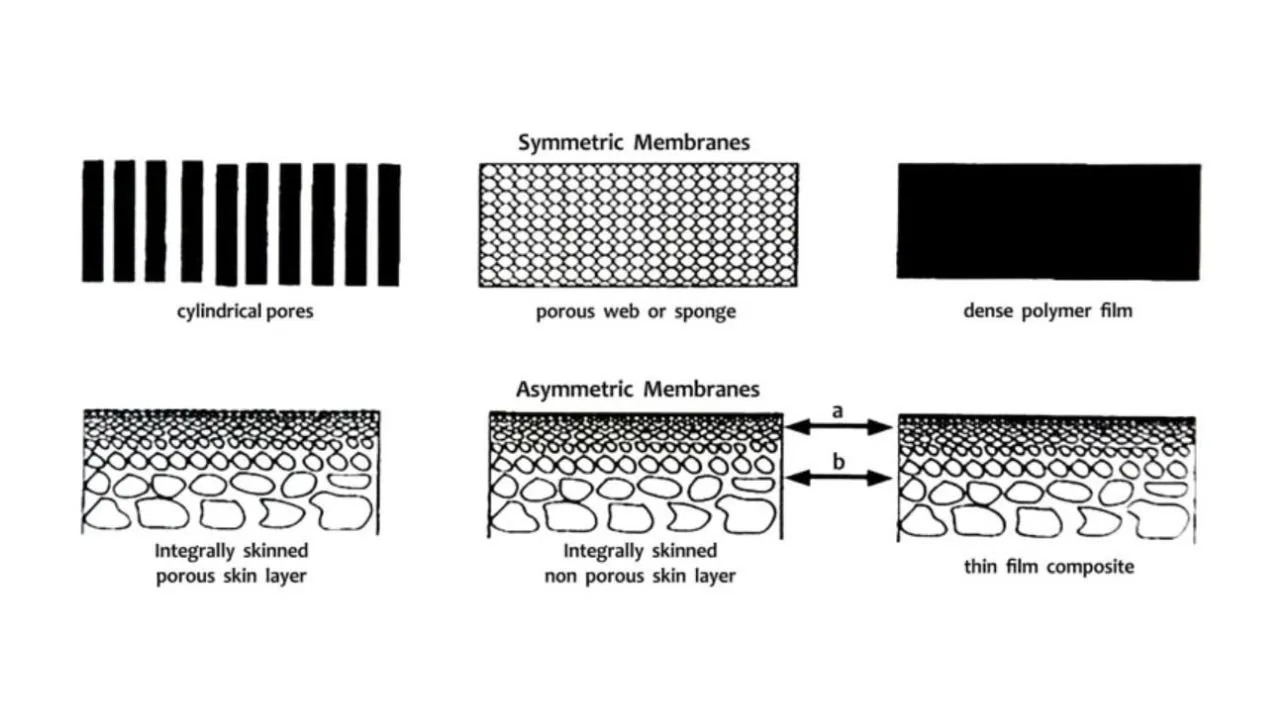
ASYMMETRIC MEMBRANES
Now, let us come to the membrane which is used for the gas separation and in this we have the asymmetric membranes. Asymmetric membranes are ultra thin (0.1 to 1 micron) high performance membrane on a much thicker highly porous support. Highly porous means 100 to 200 microns of pore size. If I have only the skin, then the skin will not be having enough mechanical stability and it may buckle. So, to prevent this buckling of the thin membrane (the skin), we need a support which is quite thick and it should not be offering any kind of resistance to the permeation of the solute. This is the reason why pore size of the supports are much bigger in comparison to the pore sizes of the skin. Thus, we have a thin skin supported on a thick support and that is why it is called asymmetric membrane.
MEMBRANES FOR NATURAL GAS SEPARATION
There are various polymeric membranes for natural gas separation-
✓ Polymeric membranes
- Polyamide (CO2 removal)
- Cellulose acetate (CO2 removal)
- Perfluoro polymer (CO2, N2, C3+ hydrocarbons removal)
- Silicone rubber (CO2, N2, C3+ hydrocarbons removal)
- Polysulfone (water removal)
PERMEATION MECHANISM THROUGH A NON-POROUS MEMBRANE
Now, let us look at the mechanism of separation through this non-porous membrane. The mechanism is called solution diffusion mechanism, which can be summarised into-
Dissolution of the gas into the high pressure upstream face of the membrane. The pressure difference between upstream side (high pressure) and downstream side (low pressure) across the membrane surface is the driving force for the solute movement. After dissolution, the gas will diffuse through the membrane. After reaching the permeate side, it has to get desorbed from the downstream low pressure side of the membrane.
PERMEATION FLUX THROUGH A NON-POROUS MEMBRANE
Now, we have to find the overall flux of the particular solute. For that, we need to consider the rate of dissolution in the membrane and rate of permeation. Rate of dissolution can be obtained from Henry’s law.
Mathematically,
CA = SA pA
Where, CA : Equilibrium stability of species A in the membrane.
SA : Solubility constant for species A-membrane pair.
pA : partial pressure of specie A in the gas.
Rate of permeation, JA = PA (pA1 - pA2)/lm
Where,
pA1 and pA2 : partial pressure of a component A in permeate and retentate
lm : Thickness of the membrane
PA : Permeability of A (PA = DASA)
DA : diffusivity of A through the membrane
Common unit of permeability is barrer.
1 barrer = 10-10 cm3 (STP) cm/ (cm2 membrane)(cm Hg)(s)
SEPARATION FACTOR
Separation factor is analogous to the relative volatility in distillation. It determines the extent to which a feed can be separated and is defined for a single stage operation having a pair of components A and B, denoted as αAB
It is basically the ratio of composition of components A (CA) and B (CB) in the permeate relative to the composition ratio of these components in the retentate.
αAB = (CA/CB)permeate / (CA/CB)retentate
MEMBRANE MODULES IN NATURAL GAS SYSTEM
We have different types of membrane modules. We can deduce them into two categories based on their uses-
✓ Spiral wound module: These are constructed using flat sheet membranes in the form of a ‘pocket’ consisting of two membrane sheets separated by a highly porous support plate.
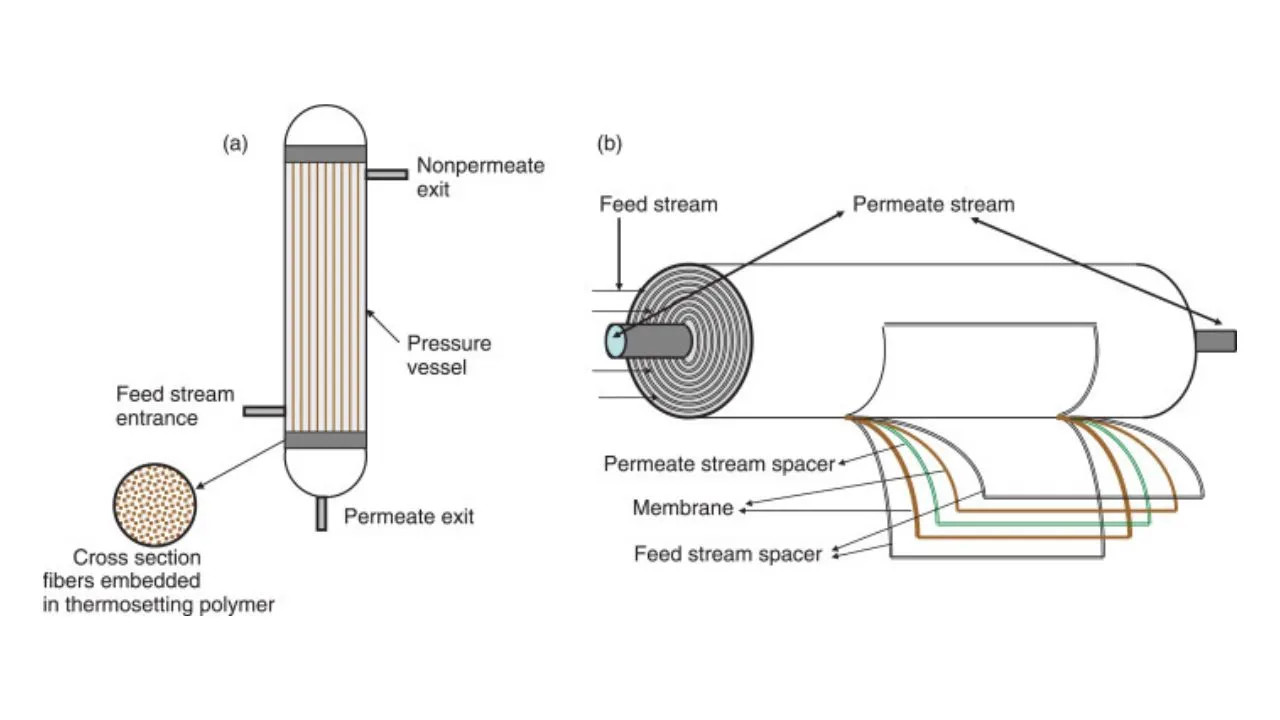
✓ Hollow fibre membrane module : These are fibers of 0.1–1 mm diameter with a hollow space inside. The feed is supplied either inside or outside of the hollow fiber and the permeate passes through the fiber wall to the other side of the fiber.
Elaborate discussion of these membrane modules are not possible on this post and also not much of importance as of now.
Membrane gas separation: A review
Review article membrane gas separation applications in natural gas processing
Membrane for gas separation and purification processes
Design of distillation column |ChemFam #15|
Separation Technique: Distillation |ChemFam #14|
Transmission Electron Microscope: Principle and Working |ChemFam #13|
Scanning Electron Microscope: Principle and Working |ChemFam #12|
Drugs: Classification and drug-target interaction |ChemFam #11|
What are orbitals and quantum numbers? |ChemFam #10|
Quantum mechanical model of an atom |ChemFam #09|
A case study about the growth mechanism of CNT |ChemFam #08|
Carbon Nanotubes (Buckytubes): Types and Synthesis |ChemFam #07|
Nanomaterials: Classification and Approach for Synthesis |ChemFam #06|
Azadirachtin: Isolation, Extraction and Mechanism of Action |ChemFam #05|
Woodward-Fieser Rules for Calculating λmax |ChemFam #04|
Chemistry in ancient India |ChemFam #03|
How do soaps clean the dirt? |ChemFam #02|
What is anti egg white injury factor? |ChemFam #01|
PS The thumbnail image is being created by me using canva.com taking template image from Researchgate

