Greetings to everyone! We shall discuss one of the important branch of inorganic chemistry, i.e., 'Organometallic Chemistry'. An organometallic compound is defined as the one that contains atleast one direct metal-carbon bond. But this excludes some carbon compounds which are considered to be inorganic such as carbides e.g., CaC2 and cyanides e.g., NaCN. The carbon containing groups may be carbonyl, alkyl, alkene, aromatic, cyclic or heterocyclic.
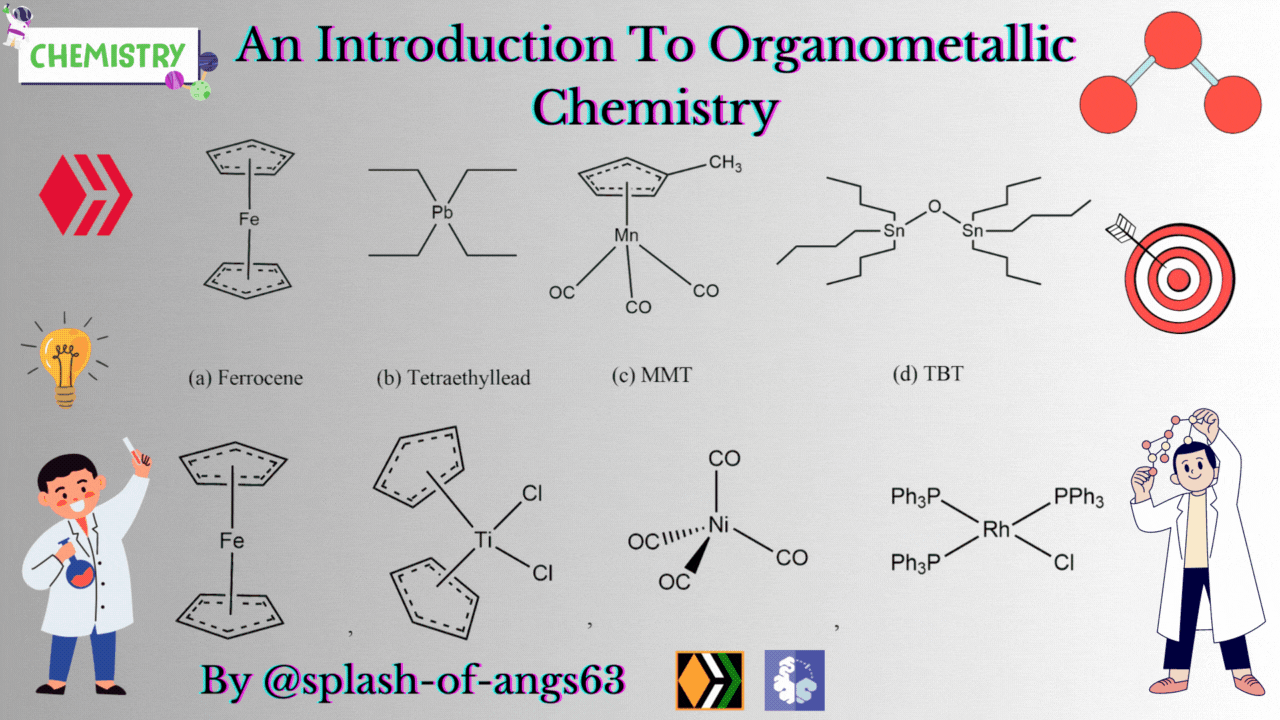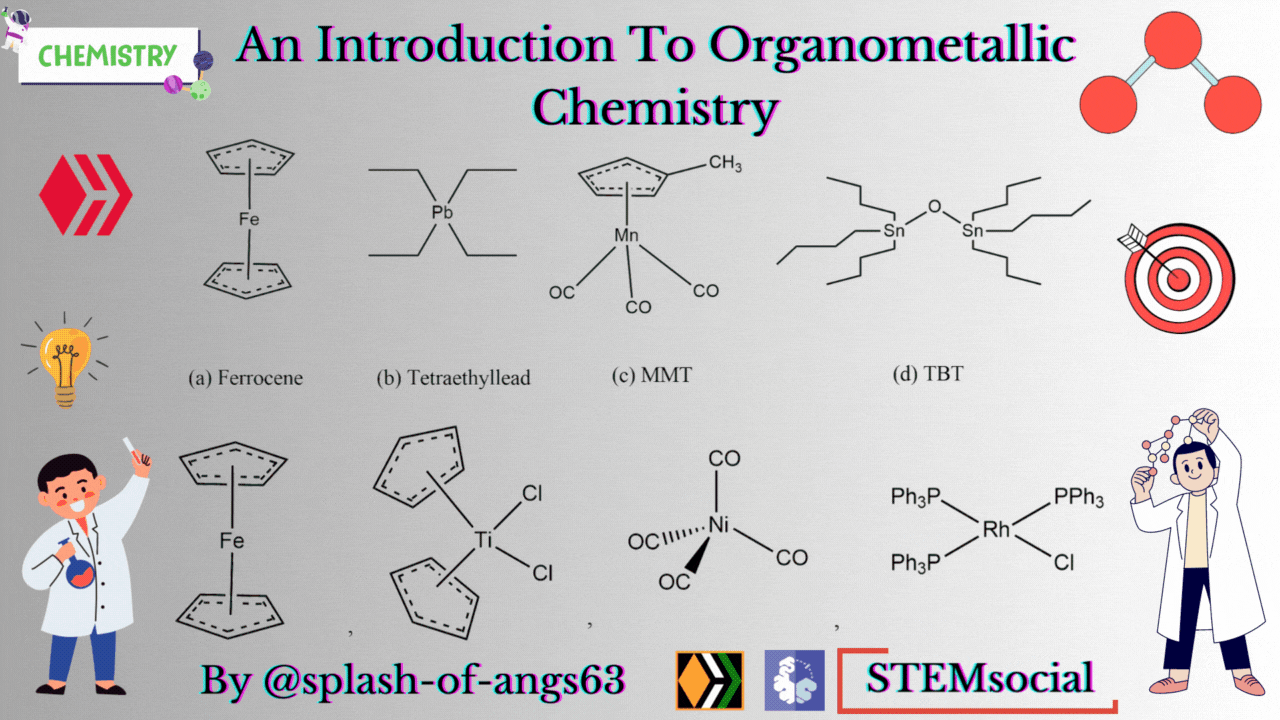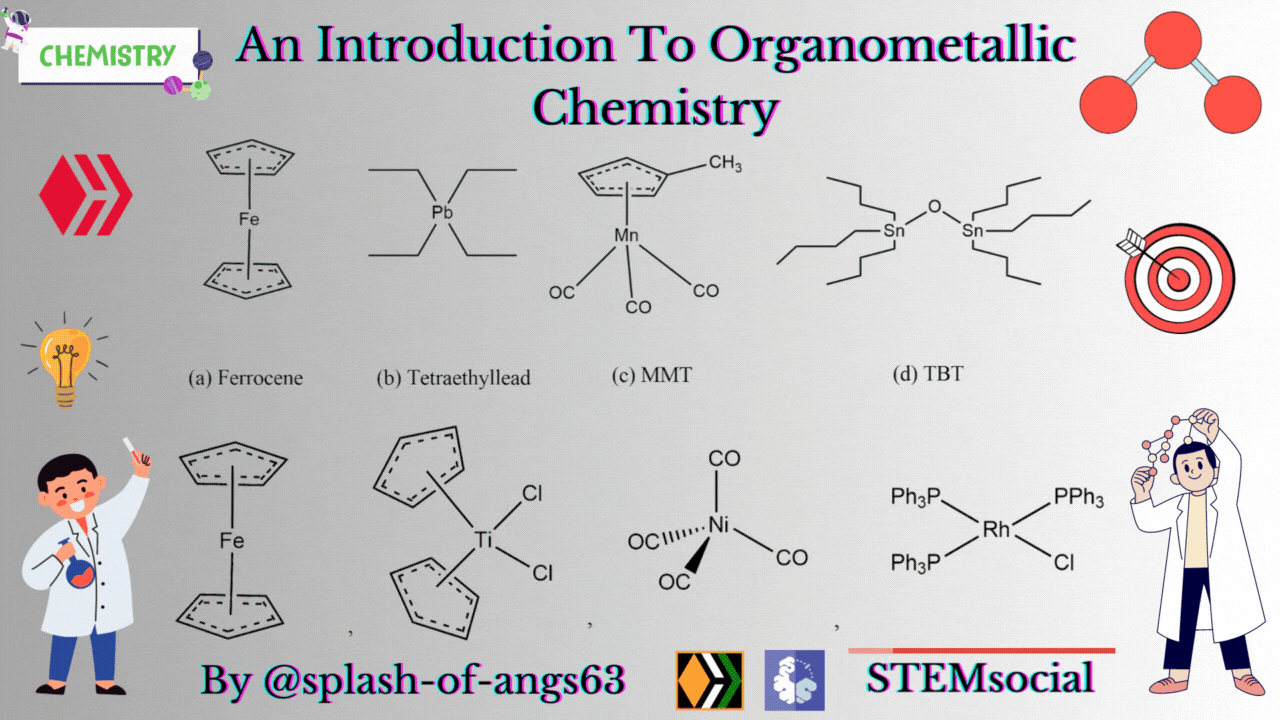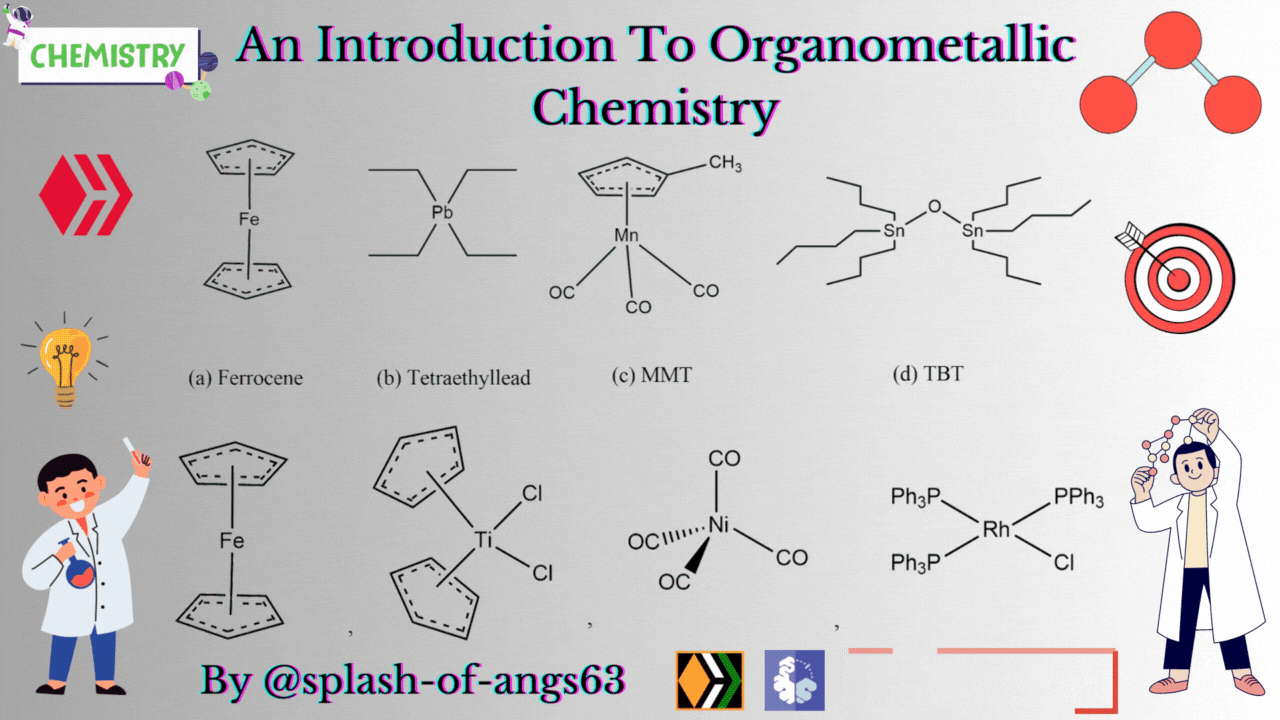
There are some complexes which actually do not contain any M-C bonds but are still considered as members of organometallic compounds. The well known example is the Wilkinson Catalyst Rh(PPh3)3Cl which is used for hydrogenation of alkenes and alkynes. In this compound, there is no direct M-C bond. However, it is an organometallic compound. To understand the reason behind this, we have to go through the mechanism. To briefly explain, during the catalytic cycle of hydrogenation of alkenes a stage comes when alkene is attached to the metal and forms metal-carbon bond. Due to this reason, it is a member of organometallic compounds. According to the leading journals of organometallic compounds, the bonding interaction is ionic or covalent, localized or delocalized between atleast one carbon atom of an organic group or molecule and a main group, transition, lanthanide or actinide metal atom. Exceptionally binary metal carbonyls like Ni(CO)4 are considred as organometallic compounds even though CO is an inorganic compound. Similarly organic derivatives of the metalloids such as boron, silicon, germanium, arsenic and tellurium are considered to be organometallic compounds.
Organometallic compounds straddle both inorganic and organic chemistry. The first organometallic compound of main group elements was cacodyl oxide which has repulsive smell and is a toxic liquid.
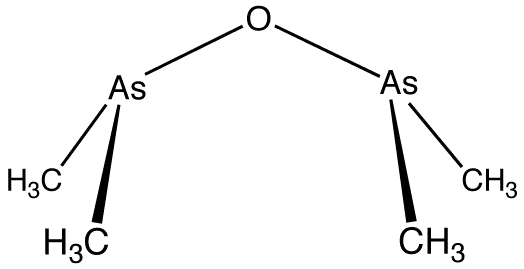
This compound was originally synthesised by heating arsenic trioxide with potassium acetate.
As2O3 + 4CH3COOK → [(CH3)2As]2O + 2K2 CO3 + 2CO2
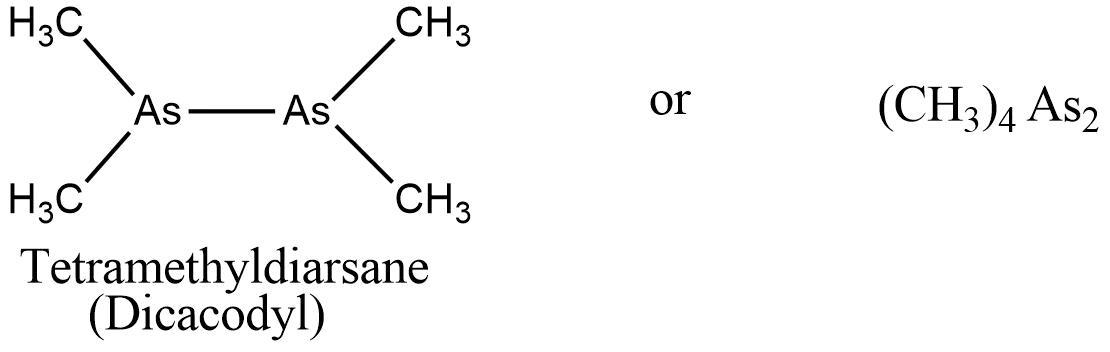
Dicacodyl is also considered one of the earliest organometallic compounds ever discovered. It was investigated by Edward Frankland and Robert Bunsen. It was originally made from arsenic distilled with potassium acetate.
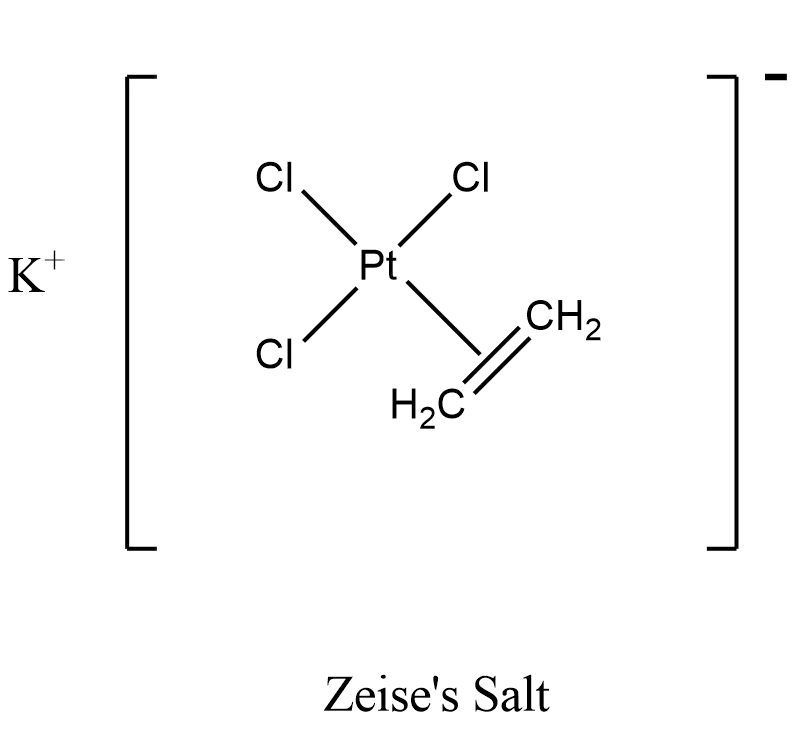
The first organometallic compound of the transition metals was Zeise's salt, K[Pt(C2H4)Cl3]
Unlike conventional transition metal complexes, the central metal atom in an organometallic compounds is often in a low oxidation state (i.e., -1, 0 or +1 or sometime +2).
On the basis of periodic table, organometallic compounds can be classified into main group, transition metal and lanthanide/actinide organometallic compounds.
Main Group Organometallic Compounds:
Examples : Na+C5H5-, n-BuLi, B(CH2CH3)3, RMgX (R= alkyl or aryl group, X= Cl, Br, I), Al2(CH3)6 etc.
Transition Metal Organometallic Compounds:
Examples,
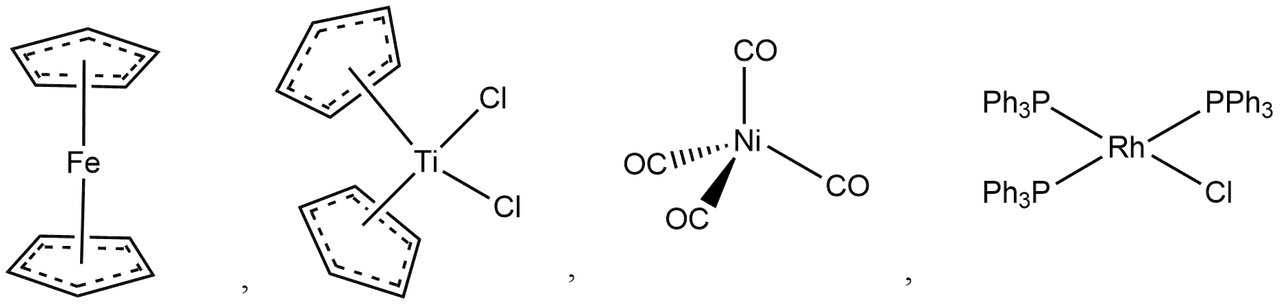
Lanthanide/Actinide Organometallic Compounds:
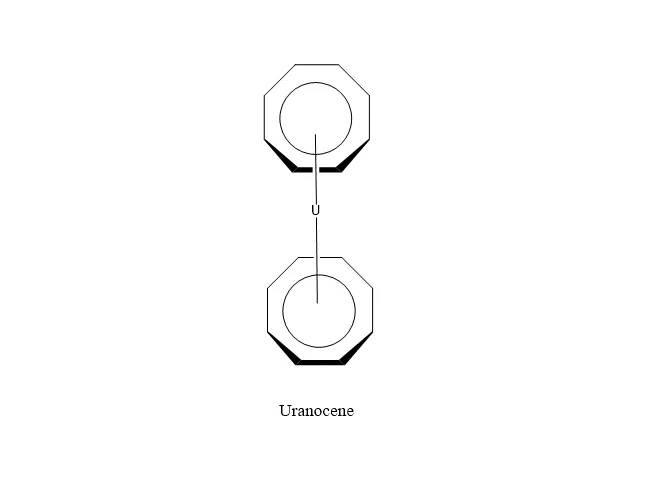
Applications of Organometallic Compounds:
(1) Organometallic Compounds as Reagents:
Some organometallic compounds specially the alkali and alkaline earth metal compounds such as n-BuLi, s-BuLi, Grignard reagent, Gilman's reagent are used as reagents.
(2) Organometallic Compounds as Catalysts:
The organometallic compounds are being routyinely used as catalysts for the synthesis of organic compounds. Some well-known organometallic homogenous catalysts are given below,
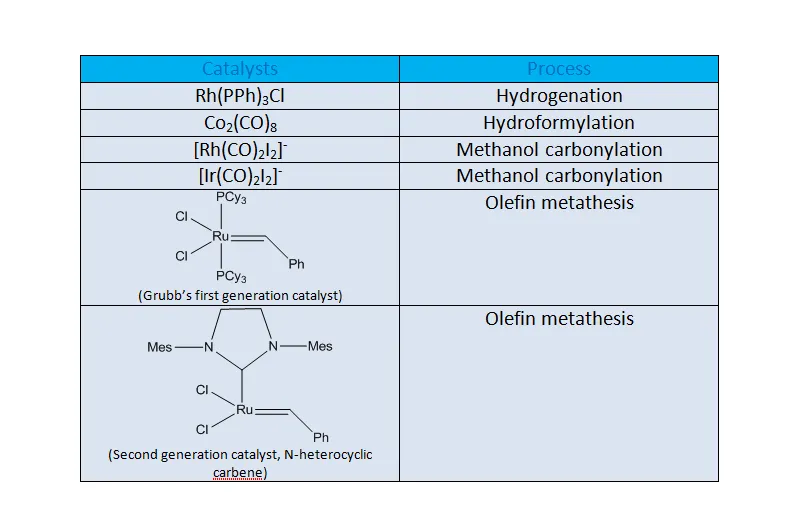
Vitamin B12 is the only known organometallic compound in nature. It incorporates cobalt ring and has been found to have many catalytic properties.
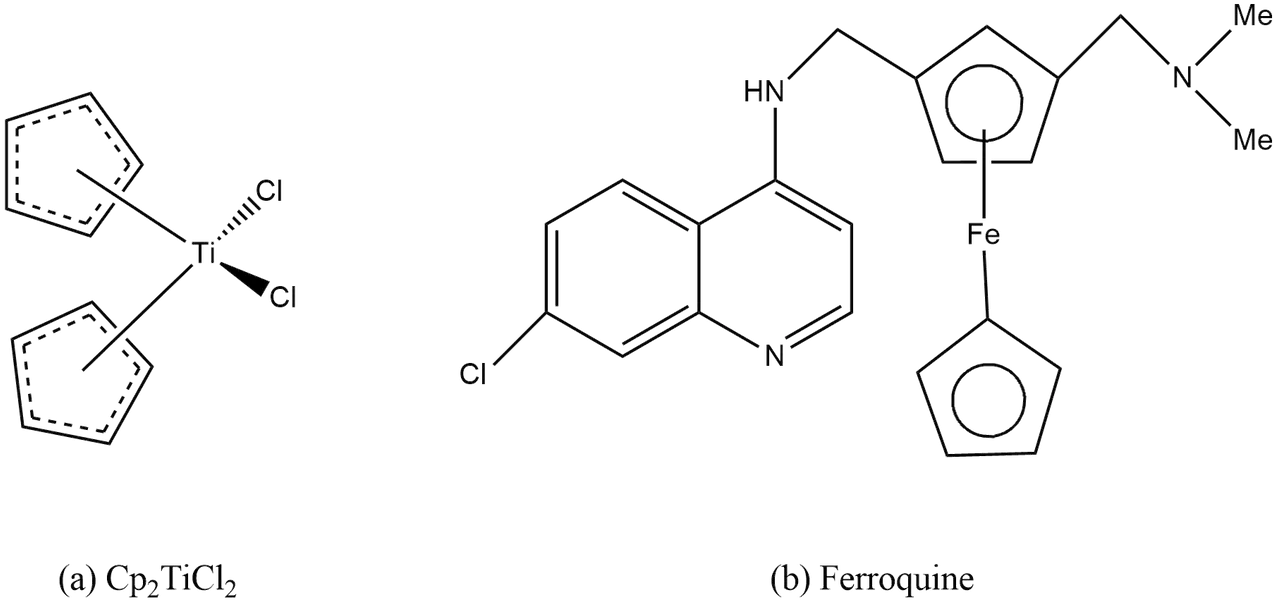
Organometallic Compounds as Drugs:
The first organometallic compound found for anticancer activity was Cp2TiCl2. An another important organometallic compound is ferroquine (FQ) which is used as antimalarial drug.
(3) Organometallic Compounds as Additives:
Organometallic compounds have found applications as bulk additives for modifying the existing properties of compounds. Ferrocene is readily soluble in liquid fuels, air stable, non toxic and usually thermally stable. Ferrocene, is therefore added to liquid fuel to reduce carbonaceous particulates are health hazards. Tetraethyllead was used as antiknocking agent in gasoline. TEL helps the gasoline to burn slowly and smoothly and prevents knocking. Combustion of TEL along with gasoline forms particles of Pb and PbO which are very harmful for health. Now, therefore methylcyclopentadienylmanganesetricarbonyl (MMT) is used in place of TEL as antiknocking agent.

It was found that MMT is harmless for automobiles. Bis(tributyltin) oxide (TBT) is an anti-foulant. It is used for coating outside of ships.
In the next post, I shall discuss about hapticity and eighteen electron rule. Happy learning!
Organometallic Chemistry (Evans)
Organometallic compounds- An overview
Applications of Zeolites: The 3D Molecular Sieves |ChemFam #34|
Properties of Zeolites: The 3D Molecular Sieves |ChemFam #33|
Zeolites: The 3D Molecular Sieves |ChemFam #32|
The Beauty of Eucalyptus Tree |ChemFam #31|
The Accidental Cure for Cancer: Cisplatin |ChemFam #29|
Acceptorless Dehydrogenation and Related Transformations |ChemFam #28|
Thermophysical Properties of Natural Gas-I |ChemFam #27|
Sources and Process Overview of Natural Gas |ChemFam #26|
Recovery, Upgradation and Purification of Helium in Natural Gas |ChemFam #25|
PS The thumbnail image is being created by me using canva.com

