Introduction
One of the growing areas of research in modern medical science is that of the role that the bacteria that live in our bodies (particularly in our gut) play in both health and disease.
Collectively known as the Microbiome, disfunction of these micro-organisms has been implicated in a whole spectrum of diseases from obesity to cancers [2].
There may also be a role in mental illness and developmental disorders.
A new study in the Journal Microbiome [1] suggests that a technique known as faecal transplantation may provide benefits in Autism Spectrum Disorders (ASD).
The study [1] is open access and you can download it here:
http://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-016-0225-7
What is Faecal Transplantation?
The formal term is "Faecal Microbiota Transplant" (FMT).
This is any technique which transfers the gut bacteria (taken from faecal matter) from one person to another.
The faecal matter is not directly transplanted but is usually treated to eliminate anything that may be considered pathogenic or harmful.
The authors use the term "Microbial Transfer Therapy" (MTT) to refer to their particular method of FMT.
I have summarised this below:
The Intervention

Here is my summary of the treatment given:
The treatment children were selected based on a 2 year physician review of their health records followed by a verification using the ADI-R (Autism Diagnostic Interview-Revised).
Children were given a 14 day course of antibiotics (vancomycin) to reduce gut bacteria.
A stomach acid suppressing agent was used from the 12th day onwards.
On Day 15 they were given a bowel flushing agent (Moviprep) to removed remaining gut bacteria.
On Day 16 participants were given a mixture of bacteria taken from "healthy" donors - SHGM (Standardised Human Gut Microbiota) either orally or rectally (see graphic).
SHGM administration lasted 74 days (10 weeks).
Children followed up for 8 weeks.
GSRS (Gastrointestinal Symptom Rating Scale) and DSR (Daily Stool Records) completed by parents to assess GI effects.
A variety of scales were used to assess behavioural symptoms, PGI-III (Parental Global Impressions), CARS (Childhood Autism Rating Scale), ABS (Aberrant Behaviour Checklist), SRS (Social Responsiveness Scale), VABS-II (Vineland Adaptive Behaviour Scale II).
Bacterial diversity was measured in stools using sequencing of microbial and viral DNA as well as visual inspections. This was then compared with controls and baseline.
Results
As the graphs show there were significant improvements in both gastrointestinal symptoms and behavioural symptoms using the rating scales.

The authors report an average 82% drop in GSRS score (indicating fewer symptoms) which was sustained at 77% level 8 weeks after SHGM therapy ended.
The PG-II rating (behavioural symptoms) showed a similar improvement with a strong correlation to the GSRS.
Core autistic symptoms went down by 22% at the end of treatment and actually continued to go down a further 2% (24% vs baseline) according to the CARS scale.
The average developmental age of the children went up by 1.4 years according to the VABS-II scoring.
No significant correlation between the improvements and age of participants was found. (Although this is a small sample and a narrow range of ages.)
Examination of the stool showed significant increases in bacterial diversity as a result of treatment:
At baseline the difference in bacterial diversity between the ASD children and the controls was quite significant. By the end of the trial:
"the distance between the recipient and the donor bacterial community was less than normal interpersonal bacterial
community variation (in this case, defined by variation between the neurotypical controls)"
So basically at the end the bacterial diversity was indistinguishable between the two groups.
Problems

Here is a summary of the main problems that I noted. I will provide further discussion in subsequent sections.
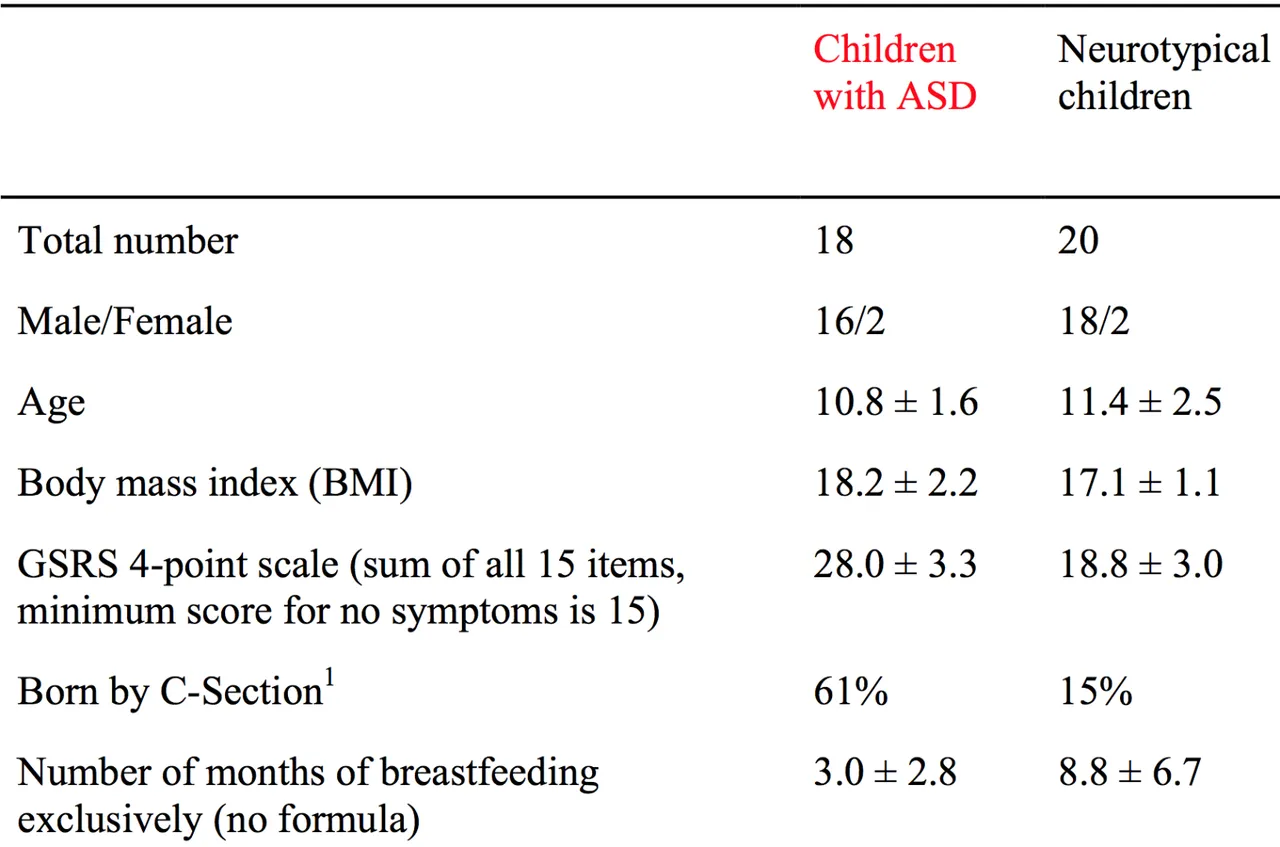
Small Sample Size. Only 18 subjects were used for FMT.
Sample was mainly male - M:F (16/2 for intervention, 18/2 controls). See additional files in paper for details.
Open Label Study - i.e. no blinding (see discussion below).
Control consisted of "neurotypical" (non-autistic) children without any intervention.
Narrow range of subject ages from 7-16 years.
Relatively short study to assess effect of intervention - 8 weeks after completion of SHGM therapy.
Was it the SHGM or the antibiotic or various other treatments that was used that caused the effect?
How objective are the rating scales being used? How does the lack of blinding and expectation affect them?
Open Label and Blinding

In medical research "blinding" [4] refers to masking the type of treatment used from either the participants or the researchers.
In single blinding the participants are unaware of the treatment being given. In double blinding neither researchers nor participants are aware of the treatment being used.
On the other hand in open label [3] trials both groups are aware of the treatment being used.
Why is Blinding Important?
It helps to reduce bias that may be introduced through expectation, suggestion or the placebo effect.
Unfortunately in some cases for practical reasons blinding can be difficult or impossible to achieve in certain cases.
There may also be ethical constraints due to the treatment being used.
In such cases an open label study may be the only option.
Discussion
I wanted to go into a little more depth as regards some of the main issues with a study of this type:
Blinding and Follow-Up

One of the biggest problems is the lack of blinding in the study.
As explained above this can introduce expectation and placebo, both of which can compromise the results.
The limited (8 week) follow up time may also add to this problem.
ASD is a lifelong condition so one could say that 8 weeks is not sufficient to determine if the changes are long lasting or even permanent.
Rating Scales and Controls

Also the use of rating scales to measure outcomes introduces it's own set of problems.
How do we know how accurate they are and how open to influence are they due to expectation?
Blinding and use of appropriate (null treatment) controls might help to assess that.
The controls here consisted of non autistic subjects who underwent no treatment.
A more useful comparison may have been to compare against ASD subjects who underwent a null treatment i.e. simulated transplant of the microbiome without actual change.
For obvious reasons this would be practically and (potentially) ethically problematic but it should also be looked at in future research as it may provide a more accurate insight.
MTT vs Constituent Interventions

There is also the potential that one of the individual components of treatment and not the microbial transplant itself was responsible for the results.
This would need further research to be determined.
For example the treatment would need to be divided up in to its' constituent elements e.g. antibiotic therapy etc and administered to separate groups with controls.
Interesting Details in the Supplementary Materials
Tucked away in the supplemental findings we can find some interesting information:
Birth Method

For example we also find some differences in method of birth delivery in the ASD children vs the Controls.
61% of the ASD children were C-section deliveries, whilst only 15% of controls were delivered by C-section.
This is interesting because Caesarian section may have a significant effect on the development of the microbiome due to lack of exposure to the mothers microbiome via vaginal delivery.
Breast Feeding Duration

Further the number of months of breastfeeding was lower (3 months vs 8.8 months) in the ASD children.
This may have effects on the immune systems of the children as well as the composition of their microbiome.
Gender Imbalance
It is also of interest that only 2 (out of 18) of the ASD children were girls.
This is particularly relevant because there has been some recent controversy about how accurate the perception of autism being a predominantly male condition is [6].
Different studies [5] have cited variability in rates from 2:1 to 15:1 (B:G).
The reasons for this may be as diverse as the variation in the disorder itself, presentation and stereotyping.
Further it has been suggested that girls may be better at disguising the symptoms.
It would be useful to know if these interventions are as effective in girls with ASD as they are with boys.
That might be an interesting avenue to explore in further research.
Conclusion

The findings of this study are certainly very interesting and do suggest that the microbiome is involved in ASD.
According to the authors it was well tolerated and so could present a potentially useful treatment method.
As always, there are problems with this study which I have discussed above.
This is a good starting point for research but the next step would be to look at tackling some of the inherent problems related to the study design.
The authors themselves suggest this in the final paragraph:
"A randomized, double-blind, placebo-controlled study is the next step to investigate the value of MTT in treating children with ASD and GI problems."
According to one of the study authors, Ann Gregory, in Medscape [7] they have already started working on a larger phase 2 study along these lines.
This is the next step on the road towards getting FDA approval for MTT as a treatment for ASD.
References
Kang, Dae-Wook, James B. Adams, Ann C. Gregory, Thomas Borody, Lauren Chittick, Alessio Fasano, Alexander Khoruts, et al. 2017. “Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study.” Microbiome 5 (1): 10.
Foxman, Betsy, Sandra Melnick Seitz, and Richard Rothenberg. 2016. “Epidemiology and the Microbiome.” Annals of Epidemiology 26 (5): 386–87.
Wikipedia contributors. 2015. “Open-Label Trial.” Wikipedia, The Free Encyclopedia. September 7. https://en.wikipedia.org/w/index.php?title=Open-label_trial&oldid=679873215.
Wikipedia contributors. 2016. “Blinded Experiment.” Wikipedia, The Free Encyclopedia. November 13. https://en.wikipedia.org/w/index.php?title=Blinded_experiment&oldid=749324681.
“Gender and Autism - NAS.” 2017. Accessed January 29. http://www.autism.org.uk/about/what-is/gender.aspx.
Satchell, Graham. 2016. “Autism in Women ‘Significantly under-Diagnosed’ - BBC News.” BBC News. BBC News. August 31. http://www.bbc.co.uk/news/health-37221030.
Levine, Barry, Andrew Nielsen, Linda Hoagland, Thomas Hilton, James Stein, Kathleen Paiero, Richard Fry, et al. 2017. “Fecal Transplants May Yield Lasting Benefits in Autism.” Medscape. January 28. http://www.medscape.com/viewarticle/874970.
Thank you for reading

Before you go have you filled in the Coinbase form to list STEEM? It only takes a few seconds. THIS POST shows you how.
If you like my work please follow me and check out my blog - @thecryptofiend
Tables and charts are taken from the paper and supplementary materials. All other images are taken from my personal Thinkstock Photography account. More information can be provided on request.