Ahead of the VRBPAC meeting we got the briefing document for the J&J vaccine. It looks to be a pretty good vaccine, though some caveats.
This one has a different primary efficacy endpoint than the others. The endpoints were moderate and severe Covid, rather than just symptomatic Covid.
This is a one dose vaccine, where they looked at efficacy at least 14 days and at least 28 days after the dose. There's also an ongoing two dose trial.
It also had several secondary endpoints including symptomatic infection, and asymptomatic infection derived from antibody testing at day 29 and day 71 post vaccination, or a positive PCR test with lack of symptoms.
It was a pretty representative trial group. They even had a person in the vaccinated group that was 100 years old. 20% were over 65. Good racial composition. 40% with comorbidities. A few HIV positive and immunocompromised people. Some participants were from Brazil and South Africa.
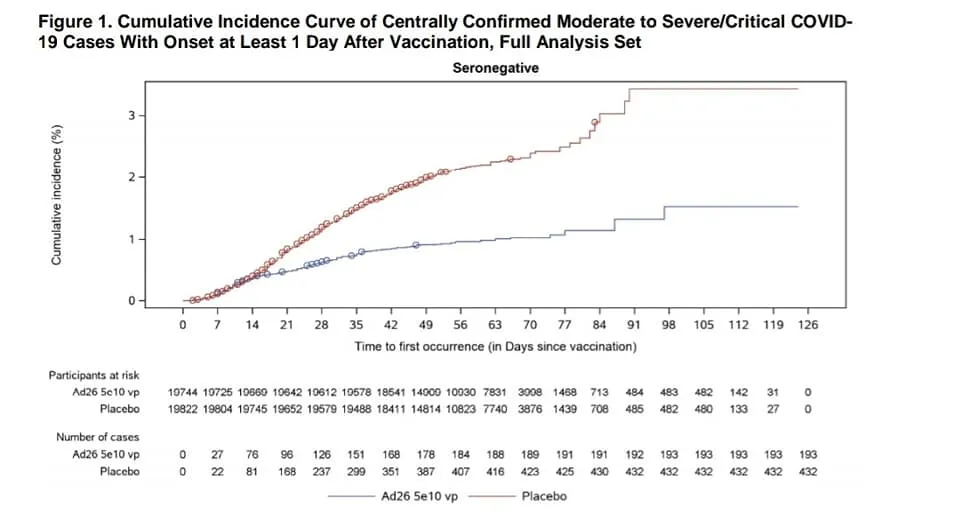
Efficacy is a bit complicated for this vaccine. This is the first vaccine we have seen the trial data for that had to deal with the new variants in South Africa and Brazil that we know impair the vaccine efficacy. And it is a one dose vaccine. With those points in mind, the efficacy numbers are better than they appear.
Like the other vaccines, efficacy seems to start to appear at day 14, but with this vaccine the best efficacy is around day 28 or later.
Against severe Covid this vaccine works quite well, even where the new variants are present. After day 28 there were no hospitalizations seen in the vaccinated group in either country in the trial.
And it appears to have good effect on asymptomatic infection, which is great news.
As far as strains in the trial, sequencing isn't complete yet, but the South African variant was predominant in the South African group. The Brazilian group had the P2 variant in about 30% of participants, but so far no P1 variant of Manaus. But the P2 variant does have the E484K mutation which is considered an escape mutation and most relevant to the vaccine efficacy. This mutation is also present in the South African variant.
As far as safety, this vaccine has a similar safety profile to the others. Similar symptoms are seen after vaccination, mostly headache, arm pain, fever, and fatigue. Tinnitus was an odd side effect seen in 6 vaccinated persons and 0 placebo, but it was mild and improved or resolved for all.
There were 4 people that got pregnant among the vaccinated and no adverse effects seen. J&J also tested its vaccine in rabbits before and after pregnancy.
I think this vaccine will be perfect for some particular groups, like the homeless, teachers, and young, healthy people, where its one dose nature and easy storage make it ideal for mass vaccination.