
By Thorwald: A-DNA", "B-DNA", and "Z-DNA
Hello again and welcome to another series of our blog post I remember doing our secondary days when our biology teacher talked about DNA, It was so so much fun and intriguing we all wanted to know about this topic, he made examples with the student in form of an experiment. Anyway we will look at the study of nucleic acids you will also learn about their significance, nucleotide is the basic monomeric unit of nucleic acids DNA is the genetic material. Genes of eukaryotes are present in ‘chromatin’ also study in details structural characteristics of DNA.
NUCLEIC ACIDS
Among all the properties of living organisms, one is absolutely important for continuance of life: a living system must be able to replicate itself. To do so an organism most possesses a complete description of itself. In living organisms this description is stored in the substances called nucleic acids. These are non-protein nitrogeneous substances made up of a monomeric unit called a nucleotide. Two different types of nucleic acids exist in living organisms, namely deoxyribonucleic acid or DNA and ribonucleic acid RNA. The monomeric unit of DNA is deoxyribonucleotide while that of RNA is ribonucleotide. The details of these nucleotides have already been discussed.
DEOXYRIBONUCLEIC ACID (DNA)
DNA is a polymer of deoxyribonucleotides and is found in chromosomes, mitochondria and chloroplasts. The nuclear DNA is found bound to basic proteins called histones. DNA is present in every nucleated cell and carries the genetic information. It is conveniently isolated from viruses, thymus gland, spleen, leucocytes, etc. Briefly, the tissue is homogenizes at neutral PH and centrifuged first at low speed to get the nuclear pellet. The pellet is then extracted with 2 M NaCI which extracts DNA from proteins. The DNA in solution is further precipitated with ethanol. To destroy any RNA present in the solution, it is treated with a ribonuclease which destroys only the RNA leaving pure DNA in the precipitate.
By Messerwoland - CC BY -S.A 3.0, Link
Primary Structure of DNA
- Chromosomal DNA consists of very long DNA molecules (MW 1.6 × 106 to 2 × 100).
- Each DNA is a polymer of about 1010 deoxyribonucleotides.
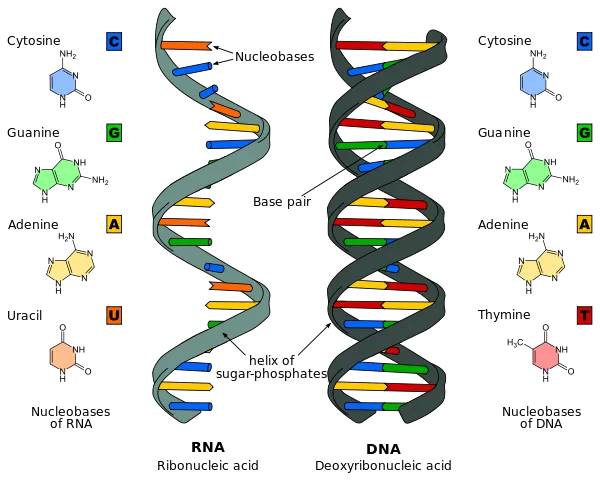
- Normally there are only four different types of deoxyribonucleotides that are found in DNA molecule, namely, adenine deoxyribonucleotide (dA), thymine deoxyribonucleotide (dT), guanine deoxyribonucleotide (dG), and cytosine deoxyribonucleotide dC).
- Nucleotides of each of the two helical strands are bound to each other by covalent 3’ – 5’ phosphodiester linkage. Each such bond is formed by the ester linkages of a single phosphate residue with the 3’ – OH (i.e., C - 3’ – OH group of the ribose sugar) of one nucleotide with the C – 5’ – OH group of ribose of the next nucleotide. This kind of bonding gives rise to a linear polydeoxyribonucleotide strand with 2 free ends on both sides.

By G3Pro - CC BY -S.A 3.0, Link
FORMATION OF PHOSPHODIESTER BOND
- That end of the strand which bears a free 5’ phosphate group without phosphodiester linkage is called the 5’ –end. The opposite end bears a free 3’ –hydroxyl or 3’ phosphate group and is called the 3’ end. ·
- The primary structure is the number and sequence of different deoxyribonucleotides in its strand joined together by phosphodiester linkages.
- The backbone of the primary structure is the linear strand of inter-connected sugar phosphate residues while the sugar residue projects laterally from the backbone.
Secondary Structure of DNA
- This consists of a double stranded helix formed is the two polydeoxyribonucleotide strands around a central axis. This type of model was first proposed by

Watson and Crick in their paper is Nature in 1953. Later on they were awarded the Nobel Prize in 1962 along with Maurice Wilkins.
- DNA is a double helix. Each of its two strands is coiled about a central axis, usually a right handed helix. The two sugar phosphate backbones wind around the outside of the bases like the banisters of a spiral staircase and are exposes to the aqueous solution. The phosphodiester bonds in the two interwoven strands run in opposite directives. Therefore the strands are called antiparallel. Thus polarity of the two strands will be 3’ – 5’ and 5’ – 3’. The 3’ – 5’ strand is called noncoding ‘strand’ .
- The aromatic rings of bases are hydrophobic and they are stacked in the interior, nearly perpendicular to the long axis of the helix.
- Adenine base of one strand of DNA is hydrogen bonded to a thymine in the opposite strand; while the guanine is hydrogen bonded to a cytosine.
- The hydrogen atoms in the bases of DNA can shift from one ring nitrogen or oxygen atom to another. These proton shifts called ‘’ tautomerisation reactions’’ – interconvert the positions that can serve as hydrogen-bond donors and acceptors in base pairs. There are two hydrogen bonds present between Adenine and Thymine while three hydrogen bonds present between Guanine and Cytosine.
- The ratio of purine to pyrimidine bases in the DNA molecule is always around 1 (i.e., G + A/T+ C=1). This is known as Chargaff’s rule.
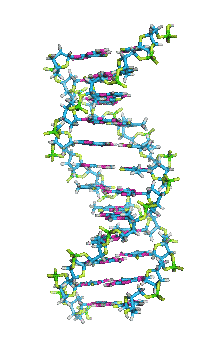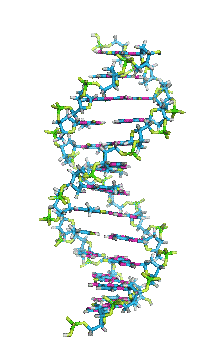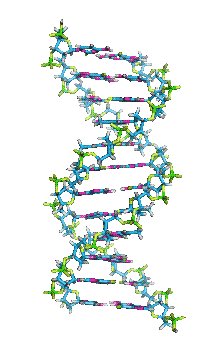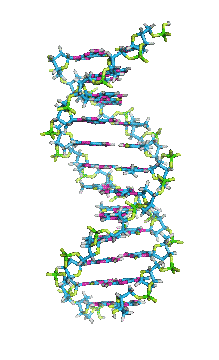
- In DNA, the glycosidic bonds between sugar and bases are not directly opposite each other and two grooves of unequal width form around the double helix. The edge of the helix that measures more than 180o from glycosidic bond to glycosidic bond is called the major groove and if it is less than 180o it is called minor groove.
I hope to continue this topic with you.
Thank you all for reading till next time.
References