A water motor is a motor that derives its energy directly from water by an electrolysis process, analyzed later, it could divide the water into hydrogen and oxygen, but it takes more energy to disassemble a molecule of water than the energy that is Releases when hydrogen oxidizes to form water. In fact, turning water into hydrogen (to burn it later) would lose much of the energy, because heat always occurs in conversions.
The first law of thermodynamics states that energy is not created, nor destroyed, but conserved. Then this law expresses that, when a system is subjected to a thermodynamic cycle, the heat transferred by the system will be equal to the work received by it, and vice versa.
That is, Q = W, where Q is the heat supplied by the system to the environment and W the work done by the environment to the system during the cycle.
Water-powered cars have been the subject of numerous international patents, press articles from popular science magazines, coverage of local television news, and urban legends on the Internet, to the point that some celebrated dramatist, such as David Mamet, has inspired by them to create a theatrical piece
It has been proved that the pretensions of creation of a motor powered by water are incorrect, and some were linked with scams to investors
Generally it could be said that the water motors would produce their own fuel from water, could be hybrids that find their fuel from water or other elements of combustion
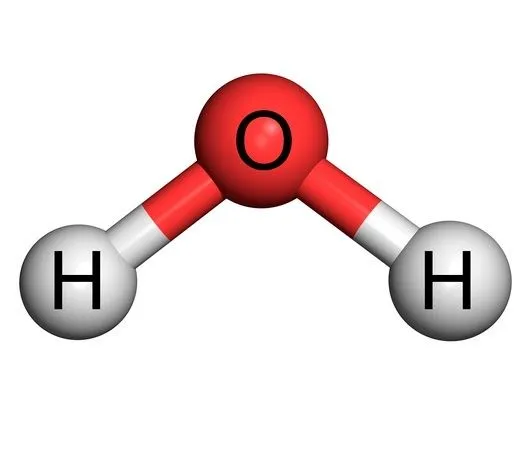
Chemical content of water
Advocates of water-fed automobiles point to the abundance and low cost of water; however, water is a chemical composed of an excess of moisture that resists most reactions.
Water does not even burn in oxygen, although it can be burned using fluorine as an electron acceptor, however, since fluorine is so reactive, most of it has turned into fluoride, and turning it back into fluoride would also require energy. For the water to participate in a reaction that releases energy, high-energy compounds should be added. For example, it is possible to generate acetylene fuel if calcium carbide is added to the water. However, in that case the fuel would not be water but calcium carbide, a high-energy material.
Electrolysis
Many cars powered by water get hydrogen or a mixture of hydrogen and oxygen (sometimes called oxyhydrogen, HHO or Brown's gas) by electrolysis of water, a process that must be powered by electricity. Then the hydrogen or oxyhydrogen is burned, providing energy to the car and also supposedly providing enough energy to electrolyze more water. The overall process can be represented by the following chemical equations:
2H2O → 2H2 + O2 (electrolysis step)
2H2 + O2 → 2H2O (combustion step)
Since the combustion stage is the exact reverse of the electrolysis stage, the energy released in combustion is exactly equal to the energy consumed in the electrolysis stage.