How much do protons, neutrons and electrons weigh?
Protons and neutrons weigh 1 atomic mass unit each. An atomic mass unit (amu) is a tiny fraction of a gram. This is about 1.66 x 10-24 gram, a reaaally small number!
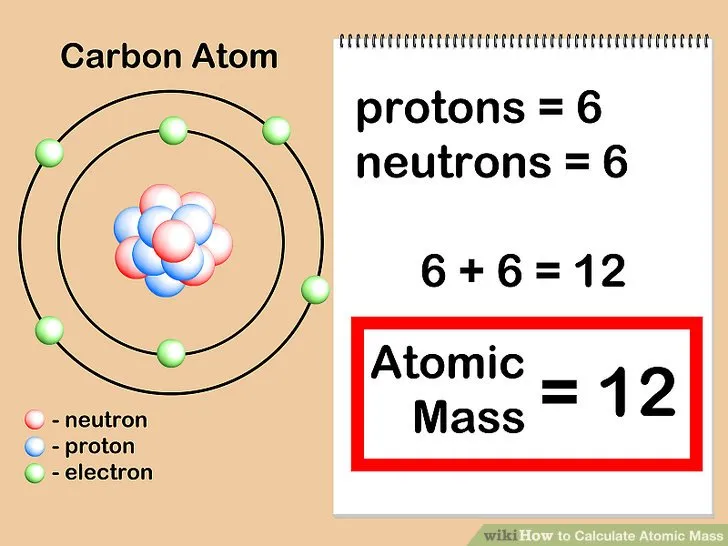
Why this weird fraction of a gram and not any other number? Because the atomic mass unit is defined using the most common isotope of carbon, Carbon-12. With 6 protons and 6 neutrons, it has a mass of exactly 12 amu. So, 1 proton has a mass of 1 amu, and 1 neutron has a mass of 1 amu as well.
Electrons are even lighter than that! They are approximately 1836 times lighter than protons and neutrons. This is why their mass is quite insignificant and we usually don’t count it in our basic chemistry equations.
What is the difference between atomic number and mass number?
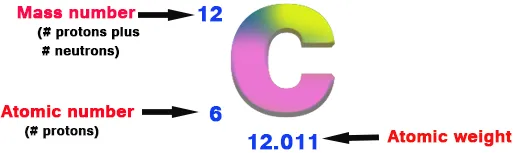
-The atomic number of an element is how many protons it has.
-The mass number of an element is how many protons plus how many neutrons it has.
So for example in the below image:
12 is the mass number (6 protons and 6 neutrons) and 6 is the atomic number (only the protons).
What is the difference between atomic mass and atomic weight?
-As we saw above, the atomic mass is the total mass of protons and neutrons in an atom or isotope.
-However, most periodic tables present the elements' atomic weight. This is the average atomic mass of all the different naturally-occurring isotopes.
As explained in a previous article, an isotope is a variant of the same element. Variants are defined by the number of neutrons. For example, the difference between the isotopes Carbon-12 and Carbon-14, is that Carbon-12 has 6 neutrons while Carbon-14 has 8 neutrons. (Note: They both have 6 protons. If they had a different amount of protons then they would no longer be carbon but another element!)
In our example, Carbon-12 is so much more abundant in nature than Carbon-14! Therefore their average (= atomic weight) is not 13 but comes down to 12.0107, which is the number you will observe on the periodic table.