
Anions and cations
Atoms can have an overall positive charge, a negative charge, or no charge at all. In a neutral atom, the amount of positively charged protons equals the number of negatively charged electrons. This means that their charges cancel out leading to a zero net charge (no overall charge).
There are cases however, in which an atom loses or gains one or more electrons. This causes the net charge of the atom to shift to negative when it ends up having more electrons than protons, and to positive when it ends up having more protons than electrons. These charged atoms are called ions.
An atom that has gained extra electrons is called an anion. Since electrons carry a negative charge, the overall charge of the atom is now negative (-). On the other hand, an atom who has lost electrons is called a cation. Since the atom now has less electrons than protons, its net charge is positive (+).
Some people confuse the two notions because they tend to associate “losing” electrons to the minus sign. Remember that the minus sign does not represent the effect of losing, but the final net charge of the atom!
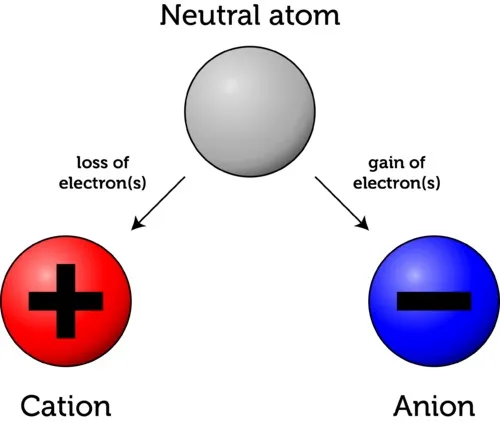
image credit
For example, if we remove one electron from hydrogen (H), it gets ionized and turns into a cation:
H+1 + e-1, or H+ + e- for simplicity.
If we add an electron to chlorine, it turns into an anion:

image credit
Ionization energy
Ionization energy is the amount of energy required to remove the most loosely bound electron of an atom. This depends on the atoms of each element, on how many valence electrons they have, as well as how many electron shells they have.
On the periodic table, the first group of alcali metals have only one valence electron each, which they are willing to give away in exchange for valence shell stability. Therefore, very little energy is required to cat-ionize them. On the other end, the noble gases are so stable that a very high ionization energy is required to remove one of their electrons and destabilize them. We realize that there is a trend on the periodic table: the ionization energy required to remove an atom’s electron depends on the number of valence electrons and thus increases from left to right.
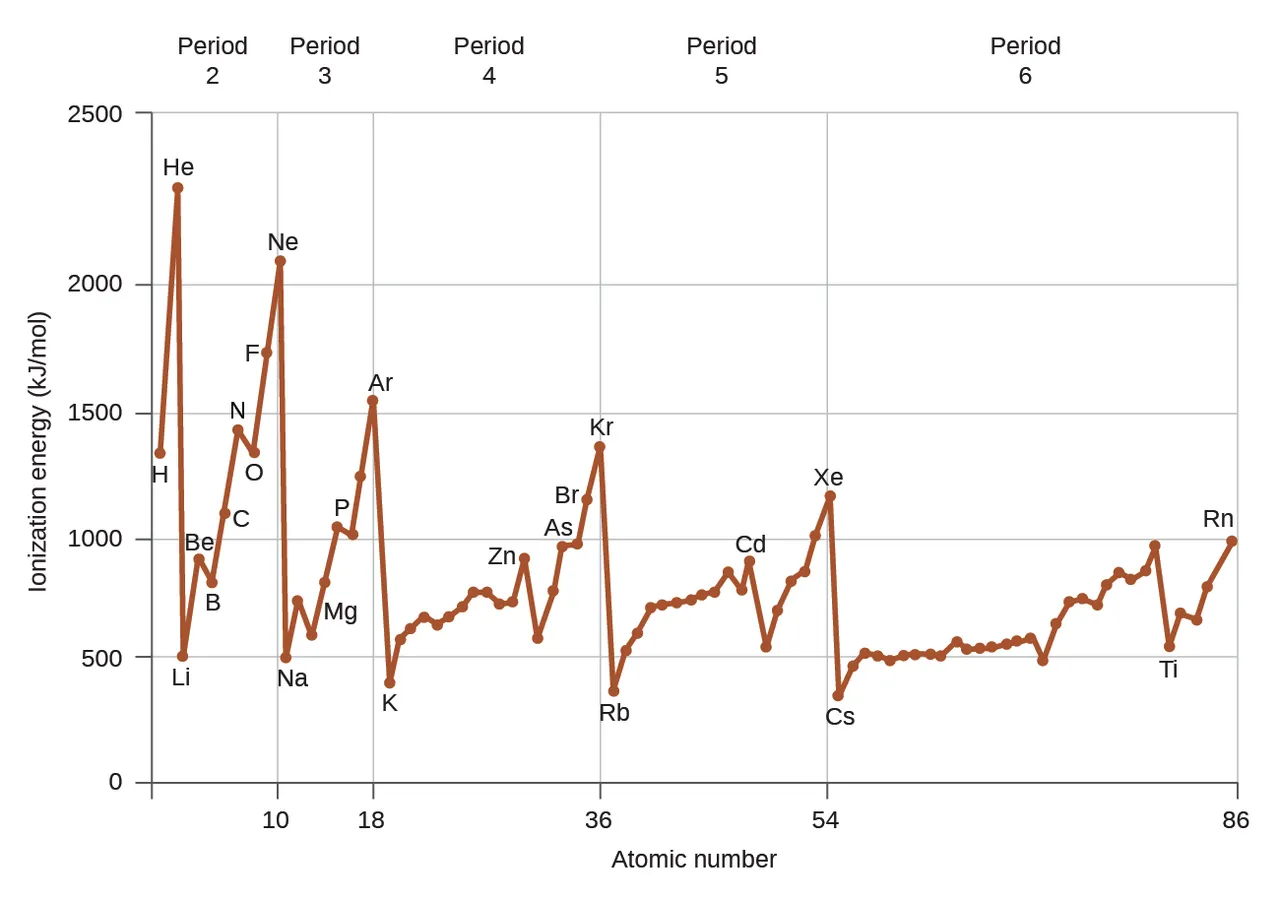
image credit
Horizontally on the periodic table, the first period comprises of small atoms with few protons and electrons. As we move downwards, more electron shells are added to the atoms of each period. The more electrons an atom has, the weaker is the attraction to the nucleus. This happens because of the electron shielding effect. Basically, the more electrons there are in an atom, the more they repel each other, because they are all negatively charged. Electrons from inner shells repel the ones from outer shells. This causes the outermost electrons to be pushed far away and the overall atom size to increase. As a result, the outermost electrons are less attracted by the nucleus and easier to remove.
Therefore, the ionization energy required to remove an electron is higher on the first periods and lower as we move to the bottom. It's important to note that for the same reason, atoms that receive extra electrons and become anions increase in size, while atoms that give away electrons and become cations, decrease in size.
Combined, these observations lead to a diagonal trend: The lowest ionization energy is required for atoms on the bottom left, and the highest for atoms on the top right.
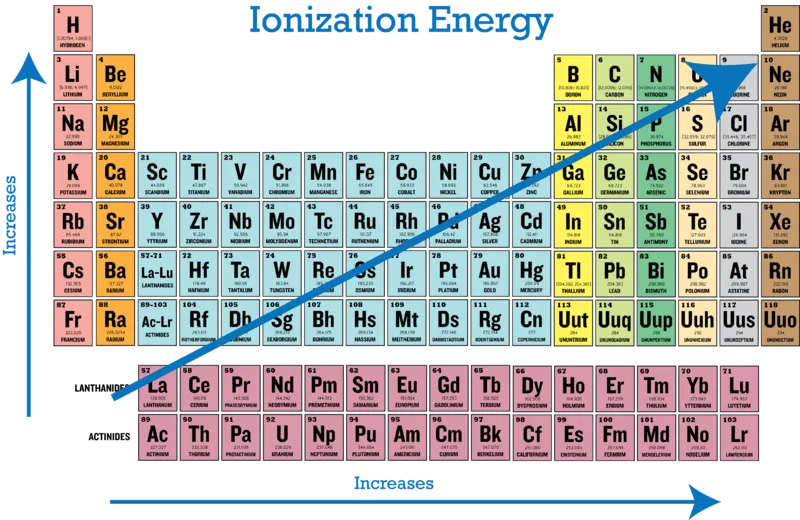
image credit
Ionization energy is measured in electron volts, which in turn can be converted to joules. Helium has the highest ionization energy of all elements, with 24.6 electron volts and francium has the lowest, with 3.8 electron volts.
