The subject of the transformation of matter has been extremely important for mankind, knowing that from this we can obtain great materials, tools or utensils that we can use to improve our quality of life.
Thus, humanity at the beginning and through observation, has presented the need to explain the phenomena that occurred in their daily lives, for example, with the discovery of fire and combustion it was possible to advance greatly in the development of the different sectors that move humanity.
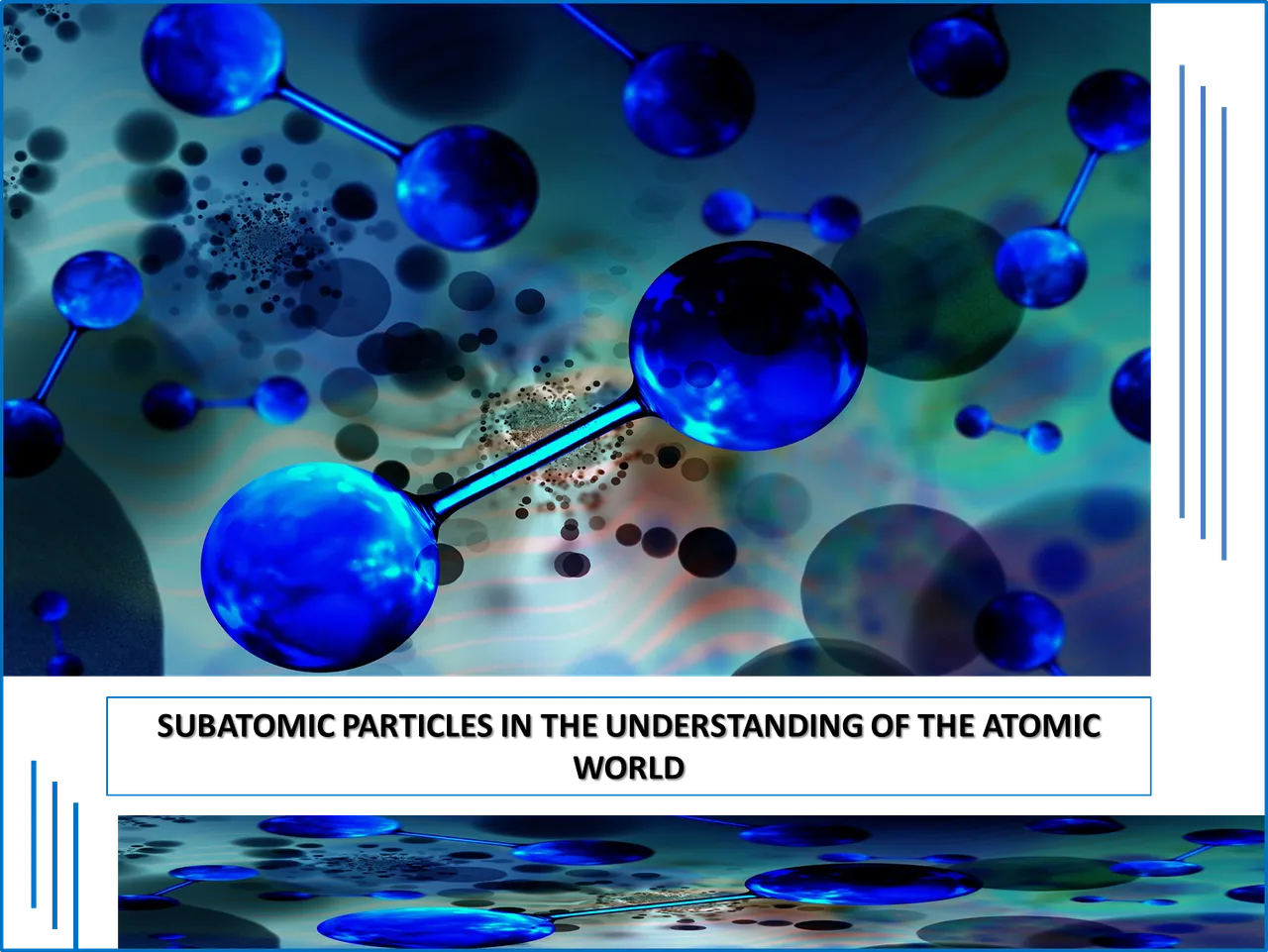
Image courtesy of: Gerd Altmann
It is also interesting to be able to explain why the reddish rock, when in contact with heat, generates a kind of material different from the starting compound, which can be implemented in the manufacture of armor and weapons, speaking specifically of iron and its great usefulness in the development.
So that the advances in chemistry and the power to justify the different phenomena that occur, has been a subject of great importance to society and that is why great philosophers of history, among which are Aristotle, Plato, Democritus, among others, have made an approach to the development of life and in their questioning it was thought that it had to come from a particular material much simpler and indivisible, ie, that could not be decomposed into smaller substances.
Consequently, people have always been fascinated by the changes that occur around us and especially by those that are generated abruptly and suddenly, hence philosophically there had always been questions about how matter is produced and what is the extent of it? Thus mentioning if it is infinite or not.
It is for this reason that from the historical and philosophical observations about matter, it was possible to make conceptual approaches to identify those substances that have an invariable composition and that cannot be decomposed into much smaller particular particles, which were called elements.

Image courtesy of: PAVM
However, it was only with the passage of time and through experimental science that we can know that the elements are formed by the combination of atoms, which are responsible for the mass of each element, so that when we talk about pieces of the same elements, it is expected that they present the same atomic mass, but when they are different, the atomic mass varies.
Therefore, to know in depth the atomic constitution it is necessary to talk about what electrons are, a concept that was first mentioned through the experiments of Thomson, this scientist of English nationality, managed to verify through his experimentation with cathode rays that small particles with negative charge traveled from one end to another with a certain energy, particles that today we know with the name of electrons.
Thomson through his research work, which consisted of a cathode tube accompanied by electrodes with positive charges (cathodes) and negative charges (anodes), was able to verify that when an electric current with a sufficiently strong voltage is applied, he is able to perceive small particles traveling from the electronegative end to the positive end and that the intensity in the movement of these small particles is directly related to the intensity of the applied charge.
Image courtesy of: D-Kuru
Also, through Thomson's studies it was established and identified that small particles could be deflected through a metal plate, which served as a barrier and the deflection was based on the intensity of the electric field and the magnetic field generated, the capacity and mass of the electron and the charge applied, so that controlling certain variables also had the ability to control the electron beam generated, which represented a more efficient use of the particles.
However, it is necessary to understand that through science and experimentation it has been determined that matter and the atoms in question are not only constituted by electrons in their structure, we can also get particles with positive charge, which have been called protons and particularly with neutral charge which have been called neutrons.
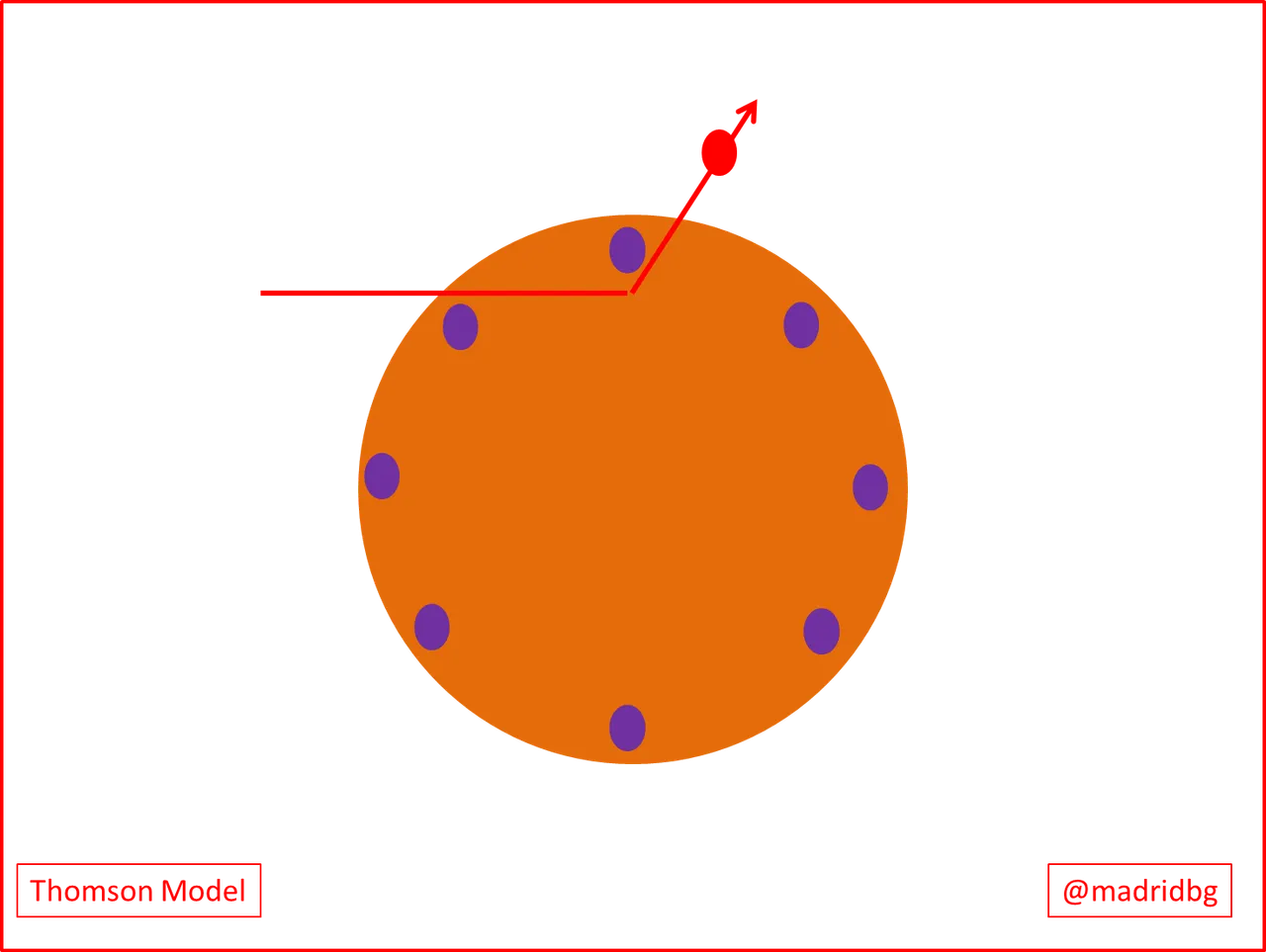
So if we focus our attention on the electronic charge, we realize that Thomson's experiment was inconclusive since in a certain way the electrons were certainly present in the atoms but at the same time they were electrically neutral, consequently this premise led the scientists of the time to think that the atoms could be visualized as a sphere where there were relationships between the charges and that beyond the negative particles there was another type of particular that provided stability to the atoms.
It was not until 1910, when the physicist Rutherford, through the use of alpha particles and radioactive materials, was able to determine that inside the atoms there was an area called nucleus, where the relationship between subatomic particles that provided stability to the atoms of the elements was found.
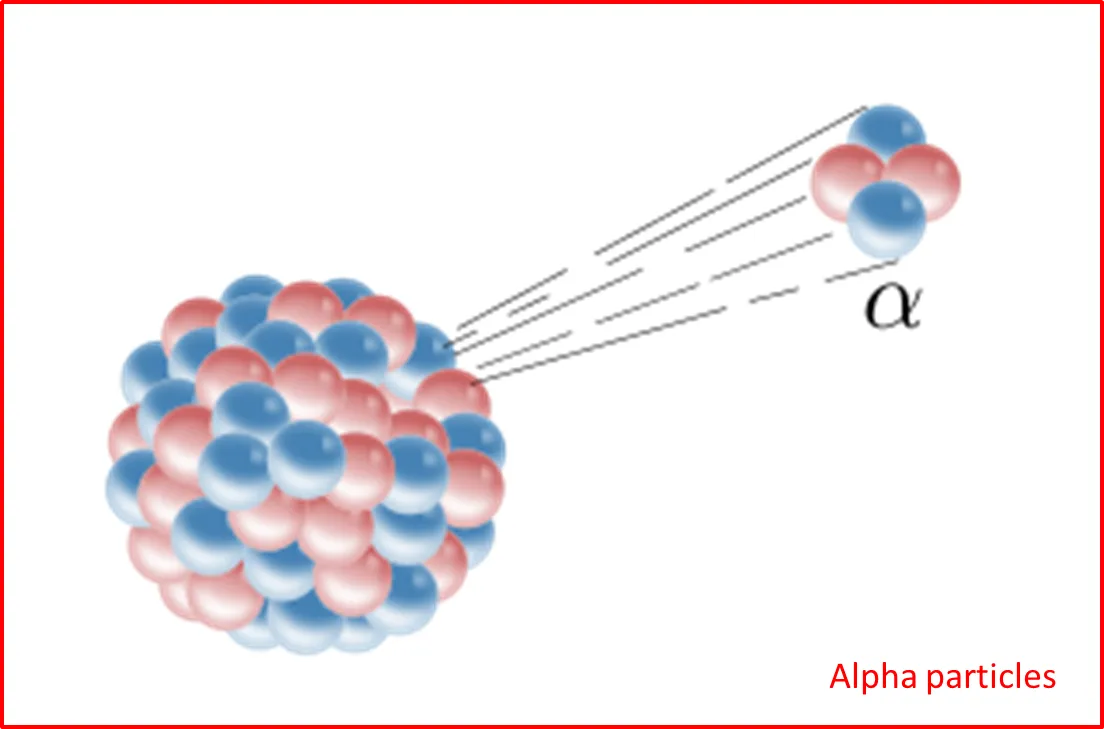
Image courtesy of: Inductiveload
These inferences were developed through a process of observation and experimentation and through the implementation of alpha particles in radioactive materials, so that the negative particles passed through a small sheet of gold and where the deviation was minimal, product of how diffuse could be the negative charges in the core of the gold particle.
Consequently, when the alpha particles collided directly in the nucleus of the material a complete opposite repulsion was observed, which could infer that these positive particles were in a central base which he called nucleus, hence the interaction of negative particles (electrons) with positive particle (protons), between the two would have to generate a charge resulting from which he called neutrons. Thus, through Rutherford's experiment it was possible to determine that in the nucleus of the elements there is a concentration of positive charges that maintain an interaction with the electrons of the nucleus.
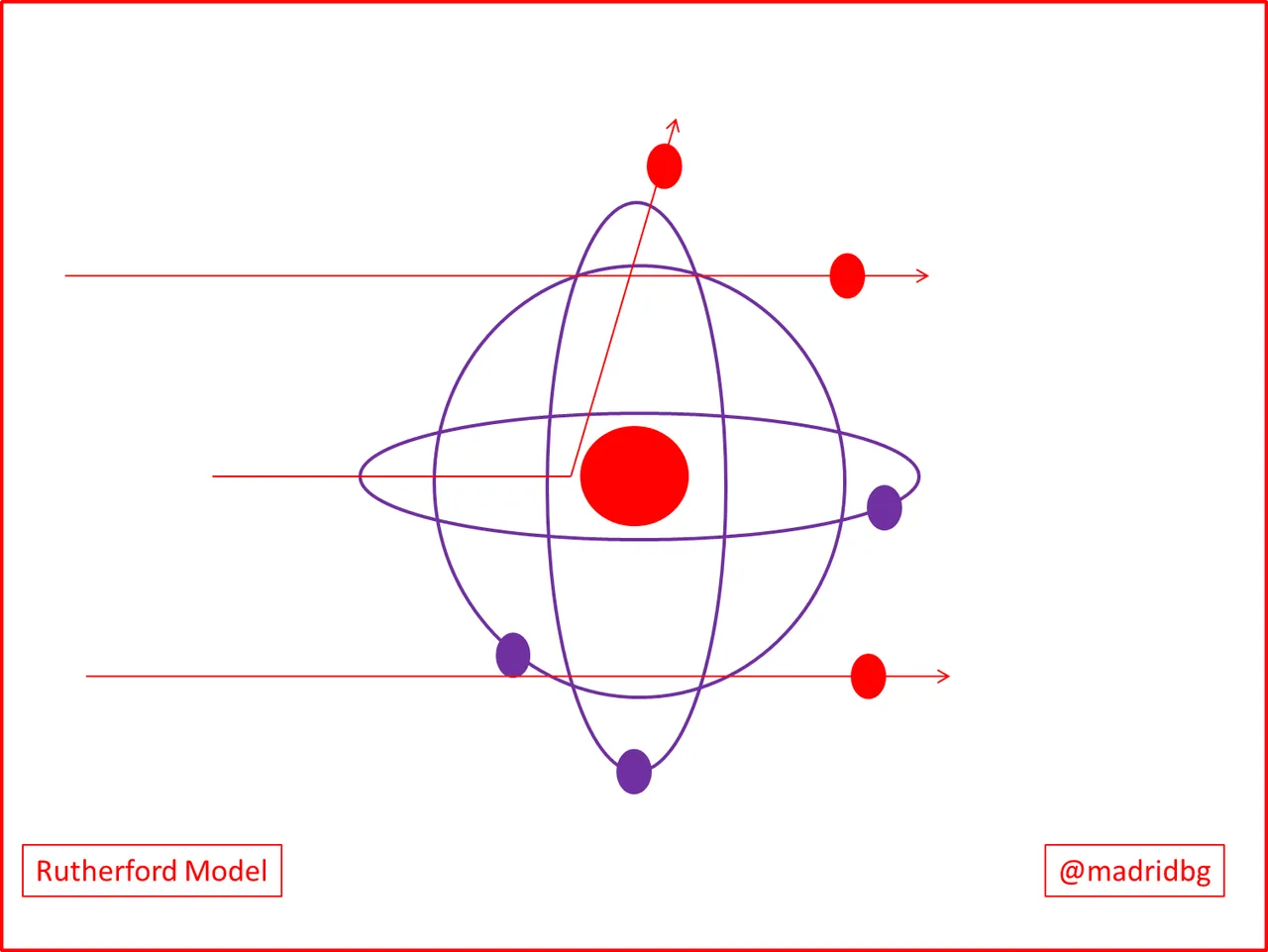
Undoubtedly through experiments they gave great advances in the scientific world since in a certain way and without having the necessary tools he was identifying the true functioning of matter, which had already been giving answers through the postulates of Dalton and Thomson and now Rutherford himself had determined and explained the foundation associated with the atomic theory.
BIBLIOGRAPHY CONSULTED
[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[3] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas
