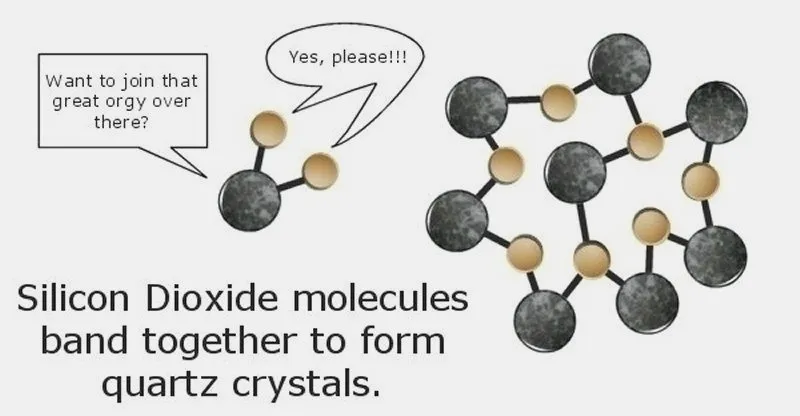
Certain chemical elements bond with others easily, such as silicon and oxygen. Silicon atoms rather enjoy having threesomes with two Oxygen atoms — one just isn't enough fun! — and the Oxygen atoms seem to enjoy it, too. When that happens, a Silicon Dioxide molecule is formed.
When many Silicon Dioxide molecules band together to form a commune, a Quartz crystal is born. As more and more Silicon Dioxide molecules join the party, the Quartz crystal grows in size.
Many quartz crystals are large-enough to be seen with the naked eye, such as this one:

Sometimes, though, something other than Silicon Dioxide wants to join the party. For instance, the monogamous Iron Oxide likes to hold hands with one of the Oxygen atoms in a Silicon Dioxide molecule.
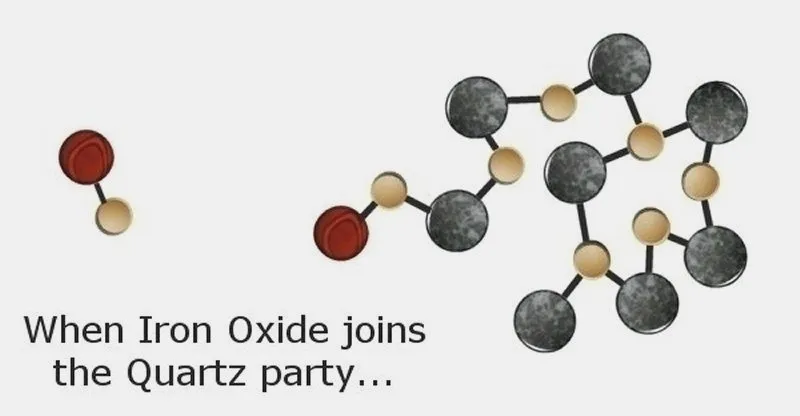
Thus, an "impurity" enters the Quartz crystal and tinges it a pinkish color. To the rock hound, though, this so-called "impurity" creates beautiful Rose Quartz!

If enough Iron Oxide joins the party, then the pinkish hue becomes absolutely red or even brown, as seen in this specimen of Red Jasper (which is a piece of Quartz, where the crystals are so tiny that a microscope would be required to see them):

The same basic principle works to create a wide range of colors in most rocks and crystals. 
———————————
See my other PrettyRocks posts 
