Aluminum is a key component in many items we take for granted today, including aircraft, electronics, kitchenware, and buildings. Although aluminum is a now a common material, it was not always so easily available. Prior to the 20th Century, aluminum was more expensive than gold and typically reserved for specialty items like military equipment. In the 19th century aluminum was so expensive that it was chosen to cap the Washington Monument. The key to making large quantities of highly pure aluminum inexpensively lay in two separate process, only one of which will be presented here. The first process, the Bayer process, produces alumina from bauxite. The second process refines that alumina into aluminum, and is the process that will be covered.

NBS (later NIST) engineers examine lightning rods atop Washington Monument (Library of Congress)
Alumina is composed of aluminum and oxygen, with the chemical formula Al2O3. The goal of refining aluminum is to separate the aluminum atoms from the oxygen atoms. There are several ways of doing this so how do we know which is the best for this particular case? Fortunately some very smart chemists and engineers of the past figured this out for us and their work is summarized in the reactivity series of metals table below.
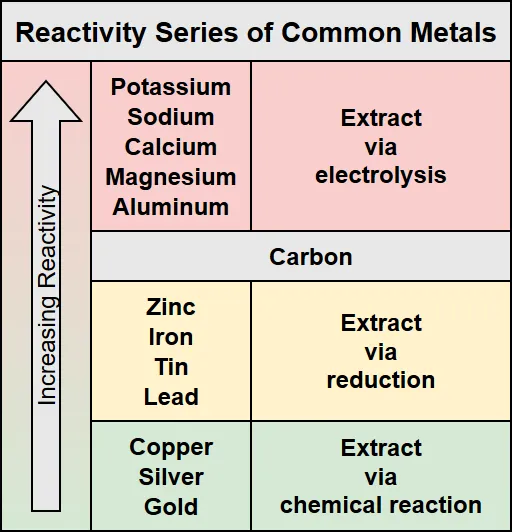
Reactivity series of common metals
Metals with a greater reactivity than carbon can be refined by electrolysis, while metals less reactive than carbon can be refined by either reduction with carbon or by chemical reactions. Since aluminum is more reactive than carbon, electrolysis is the method that will be used. However, there are several problems with trying to perform electrolysis with alumina by itself.
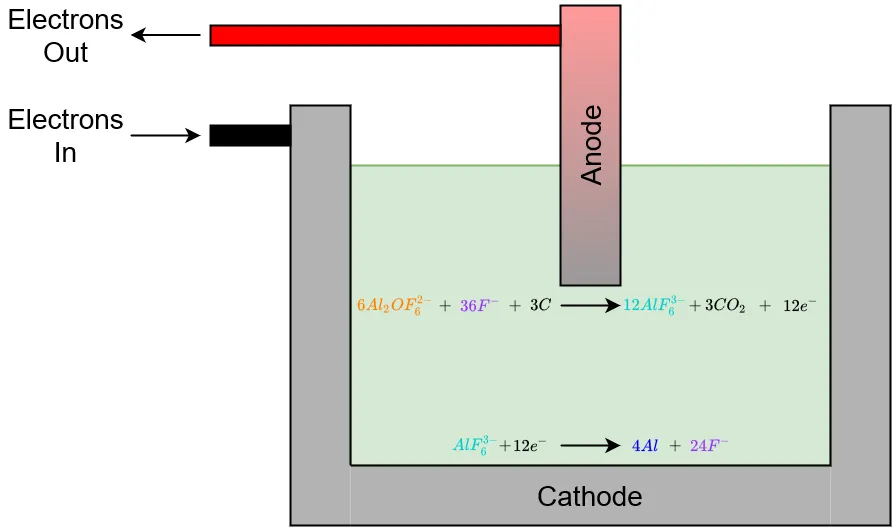
Electrolysis is a process in which electrical current flows from a cathode, through a solution, and into an anode. As electrons flow into the solution, they induce chemical reactions which ultimately free the desired element(s) within the solution from chemical bonds with other elements. The electrical conductivity of the solution is an important factor in the efficiency of electrolysis, the more conductive the solution the better the system functions. Solution temperature can also play a role in how efficient electrolysis is, highter temperatures mean less electrical energy needs to be put into the process.
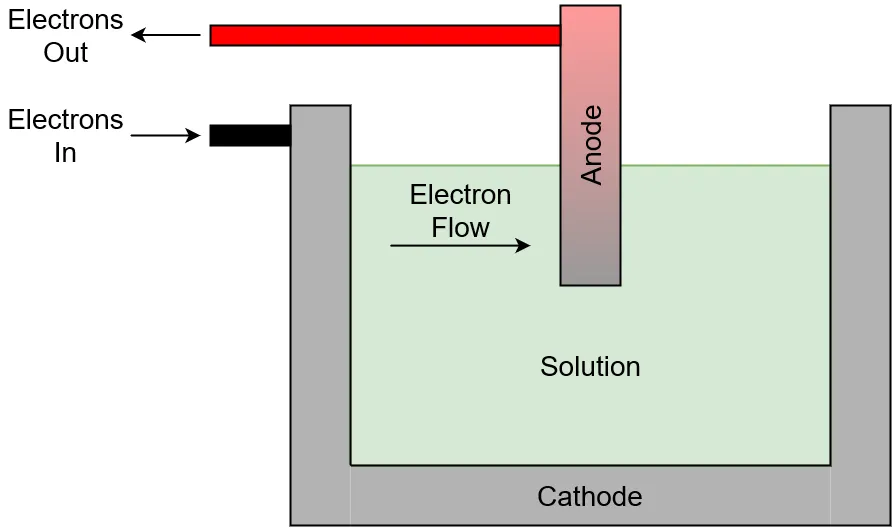
Electrolysis cell diagram
Alumina has a high melting point, but by using molten cryolite (Na3AlF6) as solvent for alumina, the melting temperature of the alumina is reduced by approximately half, to around 1000 °C. In addition to reducing the temperature required to melt alumina, cryolite also increases conductivity of the melt.
When cryolite is melted, the sodium (Na) and hexafluoroaluminate (AlF6) dissociate from each other to form sodium ions and hexafluoroaluminate ions. Each sodium ion has a +1 charge and each hexafluoroaluminate ion has a -3 charge.
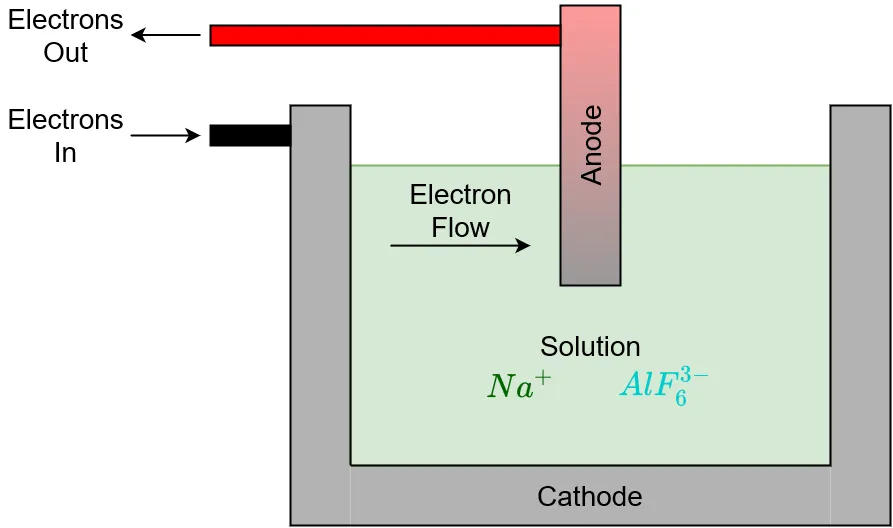
Cryolite dissociates upon melting in electrolysis cell
Upon adding alumina to the molten cryolite, a reaction occurs between the hexaflouroaluminate and alumina in which some of the fluorine atoms (F) are freed, and the aluminum atoms (Al) along with the oxygen atoms (O) combine to form Al2OF6. The exact reaction is given by

Each flourine ion has a -1 charge and each Al2OF6 molecule has a -2 charge. Because of the difference in charges, sodium ions will move towards the cathode as fluorine ions move towards the anode. Meanwhile, AlF6 molecules near the cathode will react with electrons supplied by the cathode to free aluminum atoms from fluorine atoms. This reaction is
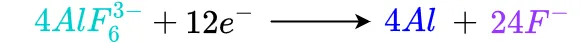
Near the anode, Al2OF6 molecules will react with the carbon in the anode as well as fluorine ions to form hexafluoroaluminate, carbon dioxide (CO2) and 12 free electrons. The electrons will move into the anode to complete the electrical circuit. CO2 is released from the solution and is vented out to the atmosphere. This reaction is given by

The density of elemental aluminum is greather than that of the cryolite/alumina solution; therefore, aluminum sinks to the bottom of the electrolysis cell where it is collected and periodically drained via a drain at the bottom of the cell.
Reactions within the electrolysis cell
To determine the overall reaction, the reaction's inputs must be balanced with its outputs using an equation-like form where similar inputs can cancel similar outputs. This arrangement also allows verification of the inputs and outputs for various reactions to make sure they balance. If an imbalance if found, the system must be checked to find errors. The form this process starts out as is
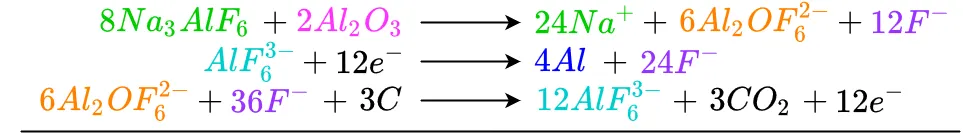
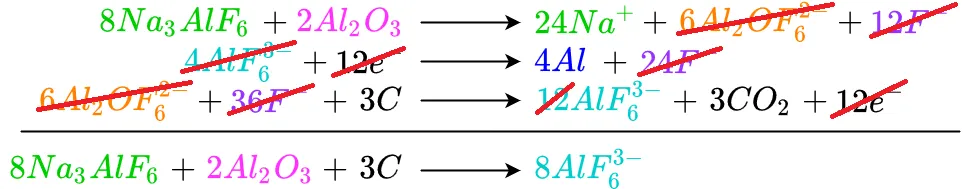
Beginning at the top, each like term is cancelled or reduced as needed. Any terms which have not been completely cancelled out are brought down to the bottom. Starting with cryolite as an input to the reaction, we see there is no cryolite in the reaction's output, so it is brought down
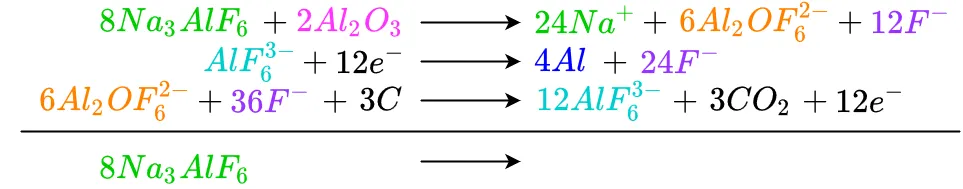
Repeating the process for alumina
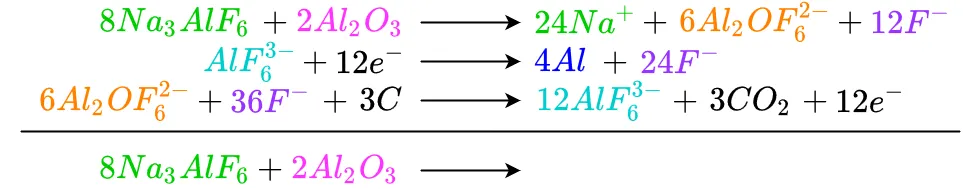
Moving down to the hexafluoroalimunate input, there is also hexafluoroaluminate on the output. Because they are unequal, the lesser is subtracted from the greater and becomes zero. The remaining hexafluoroaluminate is moved to the bottom
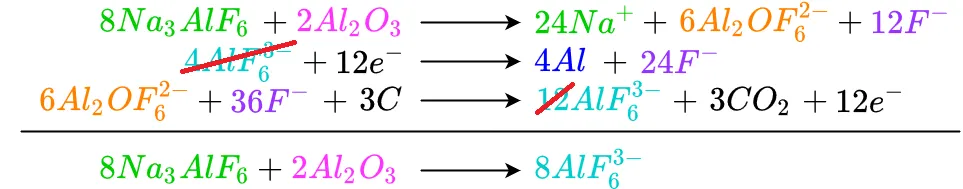
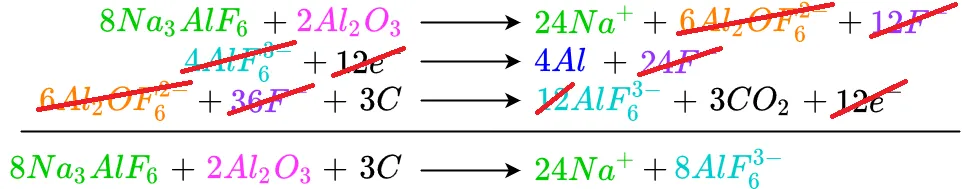
In a similar manner all the electrons cancel out
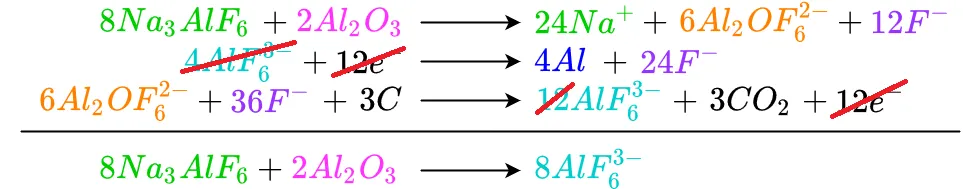
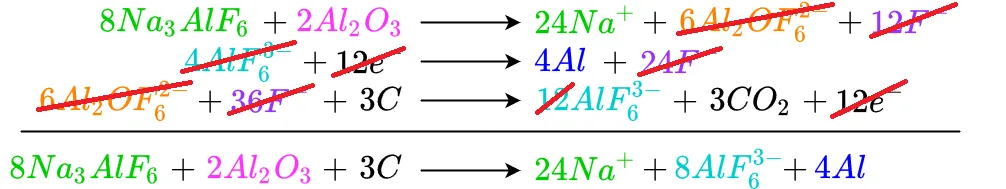
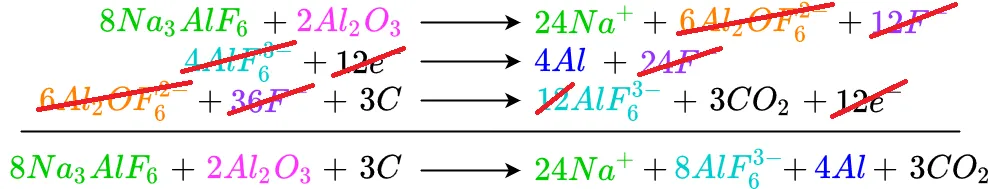
The remaining cancellations are shown below
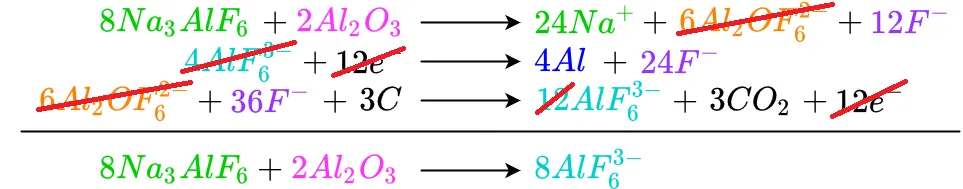
Al2OF6
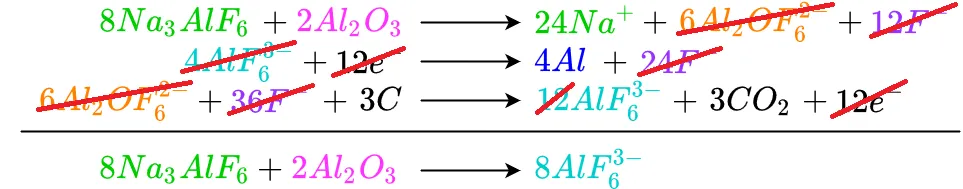
Fluorine
Carbon
Sodium
Aluminum
Carbon dioxide
Thus we see that the reaction takes cryolite, alumina, and carbon and produces aluminum and carbon dioxide. What about the sodium and hexafluoroaluminate shown in the products along with the aluminum and CO2? Examining the cryolite on the reagent side of the equation shows there are a total of 24 sodium atoms and eight hexafluoroaluminate molecules. This matches the number of sodium atoms and hexafluoroaluminate molecules on the product side of the equation. Also remembe rthatt sodium and hexafluoroaluminate dissociate from each other when cryolite is melted. If the solution were allowed to cool, the sodium and hexafluoroaluminate would recombine to form cryolite.
The amount of electricity to produce aluminum may also be estimated by looking at the number of electrons passing through the system. In this case 12 electrons move through the system for every four aluminum atoms freed from oxygen. Aluminum smelting is a very energy-intensive process, and the reason smelting facilities are often built near locations that produce a lot of inexpensive electric power such as hydroelectric dams and nuclear power plants.
Below is a video covering the process of smelting aluminum.

If you enjoyed this post, you can fine me elsewhere: