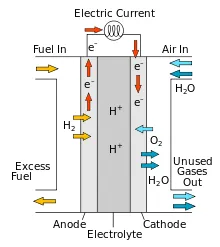
The fuel cell is an electrochemical device that is similar to a battery, but is different because it is designed to be continuously charged, the reactants are consumed; that is, he produces electricity from the supply of hydrogen and oxygen fuel from the outside. This is different from the internal energy of the battery. In addition, the electrode in the battery reacts and changes when the battery is charged or discharged, while the fuel cell electrode is catalytic and relatively stable.
The reactants commonly used in a fuel cell are hydrogen on the side of the anode and oxygen on the side of the cathode (a hydrogen cell). Usually, the reactant flow flows in and the product from the reactants flows out. So that long-term operations can be carried out continuously as long as the flow can be maintained.
Fuel cells are often considered very attractive in modern applications because of their high efficiency and emission-free use, as opposed to common fuels such as methane or natural gas that produces carbon dioxide. The only product from fuel that operates using pure hydrogen is water vapor, but there are concerns in the process of making hydrogen that uses a lot of energy. Producing hydrogen requires a hydrogen "carrier" (usually fossil fuels, although water can be used as an alternative), and also electricity, which is produced by conventional fuels. Although alternative energy sources such as wind and solar energy can also be used, but now they are very expensive.
In one example of a fuel cell, namely a hydrogen / oxygen proton exchange membrane fuel cell, a proton-conducting polymer membrane separates the anode and cathode parts. In the anode section, hydrogen diffuses into the anode catalyst.