Hello everyone, I thought today I would provide you with, my attempt at making a crappy STEM post. People message me every now and again, asking me what good content is. I don't know what the answer to that question is other than to say, good content is something that a lot of people would want to read/view/watch. Something they can't just get by a simple google search. Something with some originality, or an explanation of a complex topic not provided elsewhere...Something that you perfected yourself... Maybe you, doing an experiment yourself.. or perhaps some data that you have generated on your own... it can be a lot of things!
I can't tell you what good content is... but I can potentially show you an example of what it isn't. The following post hopefully won't be "bad," and I will do my very best to ensure that the post adheres to the (rules and guidelines) set forth by the @steemstem project. I will also offer a few decent upvotes for those who want to talk to me about this post, and tell me why it isn't so good... or argue why this is actually "quality content," and I am just an idiot. Maybe you want to yell at me for wasting your time. Or maybe in the comments you just want to shoot the shit. Talk about the weather. Post pictures of your golden retriever (my favorite dog).... that sort of thing. Up to you :)
Wait A Minute... You Can Really Power A Lightbulb With A Potato?

"And God said, Let there be light: and there was light." [1] However when He said this, He likely wasn't thinking about a potato. Yet, as one who is a true ponderer of the universe and all of the multitude of questions it presents us with... should He have been? Is a potato the source of renewable energy we have been searching for in modern times?! Could a simple spud be the answer to global warming!?!

But apparently one can use one to light up a light bulb! How does that even work? Well the answer to that question lies in electrochemistry. You see when people use a potato to power a light bulb what they are doing is making the potato into a small battery using two different metals as its electrodes. In the case of a potato battery, people typically use an OLD SCHOOL set of electrodes. Zinc and Copper. [2] Why these two metals? Well the answer becomes clear when we do some electro chemistry and draw out the half reactions for the oxidation/reduction of the electrodes [4]:
2H+ + 2e- == H2(g) 0.0 V
Total Reaction
Zn(s) + 2H+ ==> Zn2+ + H2(g) 0.76 V
So we see that when we place a piece of zinc (people often use a zinc nail) and a piece of copper (maybe like a penny?) into a potato, then connect them together with a wire and lets say... a lightbulb in the center; you create a circuit where electrons are able to travel from the Zinc electrode to the copper electrode. On the surface of the copper electrode some hydrogen ions are reduced by the electrons into hydrogen gas. If there just so happens to be a light bulb in the way those electrons will travel through the bulb and result in it becoming bright. My god! A potato is powering a light bulb! Except is it? I don't see any potato in the above reactions only metals and hydrogen ions!
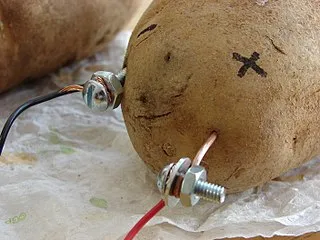
Hey What Gives, This Potato Shit Is Fake! The Potato Isn't Doing Anything!
That's not true either young padawan. See, the potato is functioning in two ways, the first of which is the role we call a "salt bridge." Which is just a substance with a bunch of ions in it that allow for the maintenance of electric neutrality in your battery. In the case of the potato battery, we have an accumulation of Zn2+ the potato helps balance that produced ionic charge. Second, the potato also has some acids in it, which help generate the Zinc ions in the first place and keep the battery chugging along. It also serves as the source for the hydrogen ions which are reduced at the copper cathode. [4]
Thank you for reading, I hope you have learned soemthing about the cosntruction of potato btatery, and powering of a lightbubl with one.
Other Cited Materials
Images
1.) A Potato, Image is public domain
2.) A Lightbulb, No Attribution Required
3.) Potato Battery Available under CC BY-SA 3.0 license
Text Sources
1.) https://www.biblestudytools.com/genesis/1-3.html
2.) http://www.physlink.com/Education/AskExperts/ae516.cfm
3.) https://web.archive.org/web/20120716205546/http://electrochem.cwru.edu/encycl/art-v01-volta.htm
4.) https://www.hunker.com/12000208/how-do-potatoes-produce-electricity
The construction of the above post did take a little bit of time. It was super boring to make though, I had very little fun. I am sure it is a real thrill to read right? You all are mega interested in potato batteries and other information that you can readily google search, or watch one of the plethora of youtube videos on right? The above explanation isn't even particularly great for how the battery works, but it sure touches the bases... and it's written in my own words. I actually went back and found some citations for the information after I wrote it so it's not plagiarized!
What should be the "SteemSTEM" curator's vote strength for this post? I mean, this is quality constructed content right? I spent a bunch of time on it. It deserves a good vote right? OR does it? What do you think?
