Greetings friends of steemit and appreciated readers.
As you know, there are many experiences in chemistry that can be developed qualitatively, so it is not necessary to use analytical grade reagents to make spectacular demonstrations that strengthen the teaching process of this science. This time I want to present a series of reactions with cupric sulfate that we can even carry out at home with accessible reagents, most of them are cleaning liquids, which gives us a way to study chemistry in a pleasant way.

Cupric sulfate crystals. Own image.
Copper sulfate, or copper (II) sulfate, is also known as blue vitriol, since the crystals of this salt have the appearance of pieces of glass with a striking blue color. For this reason it is widely used as a pigment for leathers and ceramics, but it is also a compound of great industrial relevance; It has applications in agriculture as a pesticide and fertilizer, as an algaecide in water treatment, as a textile pigment, also for producing copper coatings on other metals by electrodeposition, to name a few.
Cupric sulfate pentahydrate is a crystalline salt that has a complex structure, in the hydrated salt (CuSO4.5H2O) each copper ion coordinates with four water molecules while a fifth molecule is linked to the sulphategroup by hydrogen bonds, it goes very unnoticed, but this structure is responsible for a series of spectacular colors. Stay to read to showyou some interesting reactions with this chemical compound.
What will we use?
- Cupric sulfate pentahydrate.
- Ammonia in solution (0,1 M).
- Ascorbic acid in solution (vitamin C).
- Sodium hydroxide (6 M).
- Sodium chloride (table salt).
- Test tube and beaker.
- Heating plate.
Dehydration of cupric sulfate
In general, when referring to cupric sulfate in its crystallized blue salt form, we are talking about cupric sulfate pentahydrate, of CuSO4.5H2O formula, really the anhydrous salt of this compound has a pale white color. If we want to obtain the anhydrous salt from the cupric sulfate pentahydrate, it is enough to heat the salt directly on a heating plate, little by little the water of hydration will be lost in the form of water vapor, and gradually we will see how that blue tone goes changing to a whitish tone.

Image of cupric sulfate pentahydrate (left) and anhydrous cupric sulfate (right). Own image.
So, if the anhydrous salt is white, the blue tone is given by the water molecules trapped in the structure of the salt.
The explanation
Coppersulphate pentahydrate has a somewhat complex structure, as mentioned before, the copper ion is coordinated with four water molecules, and the fifth is linked by hydrogen bridges, this causes a distorted tetrahedral structure to form around the metal ion.
In the anhydrous salt the Cu2+ cation has the electronic configuration [Ar]3d9, so it would have nine electrons arranged in the five d orbitals, arranging them in two different levels (t2g and eg) which, roughly, due to the distortions unfold, the color change will depend on the distortion. Thus, in the anhydrous salt a tetrahedral structure is formed in which the copper atom occupies the vertices and centers of the alternate faces while the sulfur occupies the holes; but in the pentahydrate salt, copper interacts with six ligands (four H2O and two sulfates), which distorts the axial plane, as seen in the following image.
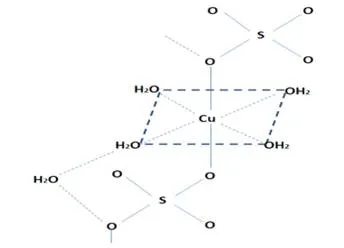
Structure of cupric sulfate pentahydrate.
In the case of cupric sulfate pentahydrate, ligands with water are weak field, which means that they cause a small variation of energy between these levels but they are sufficient to produce electronic transitions between them, which absorb radiation in the longitude wave around 600 nm, which absorbs the color orange and emits the blue color.
Obtaining copper chloride
Let's start this experience by dissolving enough cupric sulfate in water to obtain a solution of approximately 1 M concentration. If we place 2 mL of this solution (blue) and add sodium chloride (NaCl), we will obtain a green solution.

Solution of cupric sulfate and copper chloride. Own image.
The explanation
Through a double displacement reaction, copper chloride (CuCl2) and sodium sulfate are produced, the corresponding chemical equation would be:
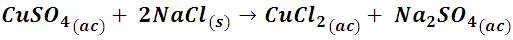
The solution turns green because the replacement of the H2O ligands by Cl- ligands, with a less intense field, causes the energy absorbed to be lower, in the range of 400 nm, with which the complex takes yellowish colors, however, complex solutions also form Cu[(H2O)6]2+ complexes in blue, the solution turns green by combining the blue and yellow tones.
Obtaining metallic copper
Metallic copper can not only be obtained from its native state in nature, but it can also be obtained from its compounds. From the cupric sulfate we can obtain it by the formation of precipitates of various complexes. If we have 2 mL of the cupric sulfate solution in the test tube and we gradually add ammonia solution, we will notice two things, 1) the formation of a turquoise precipitate (it is seen as if the solution turns turbid) and 2) that laterthe solution acquires a blue color. If we then add the ascorbic acid (vitamin C) we will observe that solution turns orange, if we let it rest we will notice that it is due to the formation of a reddish precipitate, which corresponds to metallic copper.

Images of:cupric sulphate (A), turquoise precipitate (B), blue ammoniacal complex (C) and metallic copper precipitate (D). Own source.
The explanation
With the addition of ammonia the solution takes on a gelatinous appearance due to the formation of a turquoise precipitate according to the following reaction:

However, by continuing to add ammonia solution, this precipitate dissolves forming tetraamine complex, Cu(NH3)42+, according to the following reaction,

This series of reactions introduces the formation of stronger field ammonia (NH3) ligands in the solution, whose distortion absorbs the yellow spectrum, giving rise to a more intense blue color solution.
By adding ascorbic acid to the solution, it acts as a reducing agent, yielding the necessary electrons to lead to the reduction of copper ions to metallic copper, which appears as a fine orange powder in the medium, when left to rest we can better notice its characteristic reddish color.
Obtaining copper hydroxide and copper oxide
Cupric sulfate can also generate even more intense color precipitates. If for example we have 2 mL of the cupric sulfate solution in the test tube and progressively add sodium hydroxide, we will notice the formation of a gelatinous blue precipitate, and by continuing to add sodium hydroxide, it becomes an even more intense blue. . If we then proceed to heat this mixture, we will notice that the precipitate dissolves and acquires a darker tone until it forms a black precipitate.

Image of the precipitates of: copper hydroxide (A) and copper oxide (B). Own source.
Explanation
When we add sodium hydroxide to the cupric sulfate solution, a blue precipitate and a gelatinous appearance of copper hydroxide are first produced, as described in the following reaction:

By continuing to add sodium hydroxide the precipitate acquires a more intense tone, this is due to the fact that in strongly basic solutions the formation of the cuprate ion (CuO22-), of a darker blue tone is promoted and, with heating, it dissolves the precipitate of copper hydroxide and a precipitate of copper oxide (CuO) in which is a black color obtained. These compounds have a tetrahedral and square planar structure that imply a less absorption of energy, giving complexes of dark colors (indigo and black).
References
Wikipedia.com. Copper (II) sulfate.
Heredia, S. (2006). Experiments with copper sulfate. Eureka Magazine, pp. 467-484.
Heurema group. Copper complexes.
