Hello Steemians,
So, I've been planning to start some series, but I was confused due to a number of subjects I could come up with. Finally, I've decided that I will start everything one-by-one. So here's the first series with the name "Why is it so ?". This series is inclined towards the explanation of basic fundamental things with a little more detail. I'll be doing some extensive research on the subjects before posting my own article about it. A few of the questions can be:
"Why do we need oxygen to live?",
"Why our heart keeps on beating? (Well, till it stops actually, gaawwdd wrong question)",
"Why feel certain emotions?",
"Why Jennifer Lawrence is so awesome?" (Because she's Jennifer Lawrence, duh..!),
"Why John Snow knows nothing?" (Probably because George RR Martin didn't want him to know.) etcetera etcetera so on and so forth..
So here comes the first one, "Why we need oxygen to live?", a fairly simple question. I mean, when I think about it, why oxygen specifically, and why it couldn't be anything else. After some research about the topic, I found some interesting details, which many of you already know, but I'm a dumb person and I needed to know so there..!! :D
Why we need Oxygen to Live ?
Well first of all, Oxygen is a gas, which is reactive. by reactive I mean, it can be combined with other substances to create some other substance(s). The reaction procedure changes the arrangements of constituent atoms of the elements taking part in this procedure, which eventually generates another substances as products. Oxygen is not too reactive, otherwise it would've started reacting whenever it came in contact with any periodic table element. Some well known example of Oxygen reacting with other elements are:
H2O - Water - One oxygen atom combined with two of hydrogen, the reaction generating the product called water.
CO2 - Carbon Dioxide - Carbon reacting with Oxygen generating carbon dioxide (It's called dioxide because there are two atoms of oxygen present in the molecule or empirical formula of the product)

Image Credit + Edited my Me
I've learned in science class, that two or more atoms make a bond because they exchange electrons to either possess positive/negative or neutral charge. Sometimes, the atom is so heavy, it pulls the other atom towards it, (basically it's pulling the electron of that other atom and the electron possessing atom comes right in crashing kinda thingy, that's how much I can scale it down for you to understand, okay ?)

Image Credit + Edited by Me

Image Credit + Edited by ME
But there are many other factors play a major role here and it doesn't always go single way. Atoms react with each other in various way and it is still not clear why atom choose to react in various way that they do.
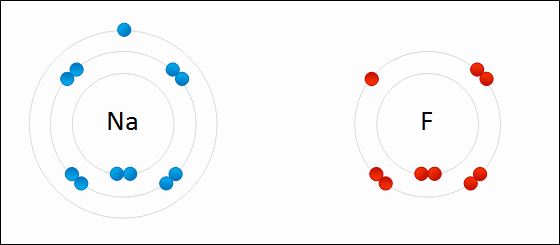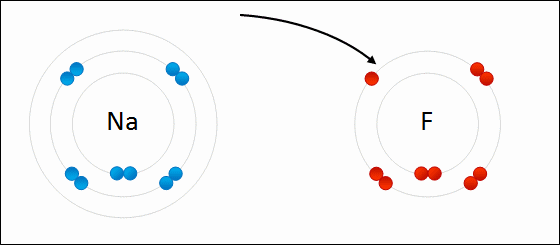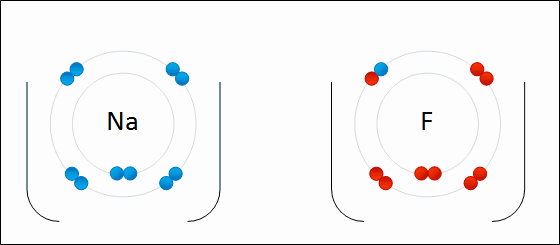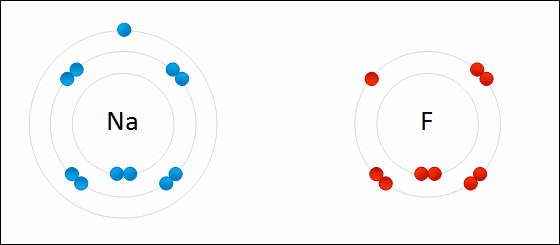
REF: http://www.meta-synthesis.com/webbook/50_why/reactions.html
So now, back to our original question. So we know that oxygen is the gas which is moderately reactive. Basically the cells in our body have their own powerhouse called "Mitochondria". They generate ATP (adenosine triphosphate) from glucose and oxygen, and this ATP is responsible for providing energy to our body (Yet so many other chemical reactions to reach this level, but eventually we'll reach to the energy generation point).

Image Credit + Edited by ME
- How ATP generates energy you may ask ?
- How ATP generates energy you may ask ?
Well ATP is adenosine triphosphate, you see - three phosphates, and every time one phosphate is released, it generates a bunch of energy for our body to go rock-n-roll.
Adenosine Triphosphate --> ADP (Adenosine Diphosphate) + Phosphate + Energy
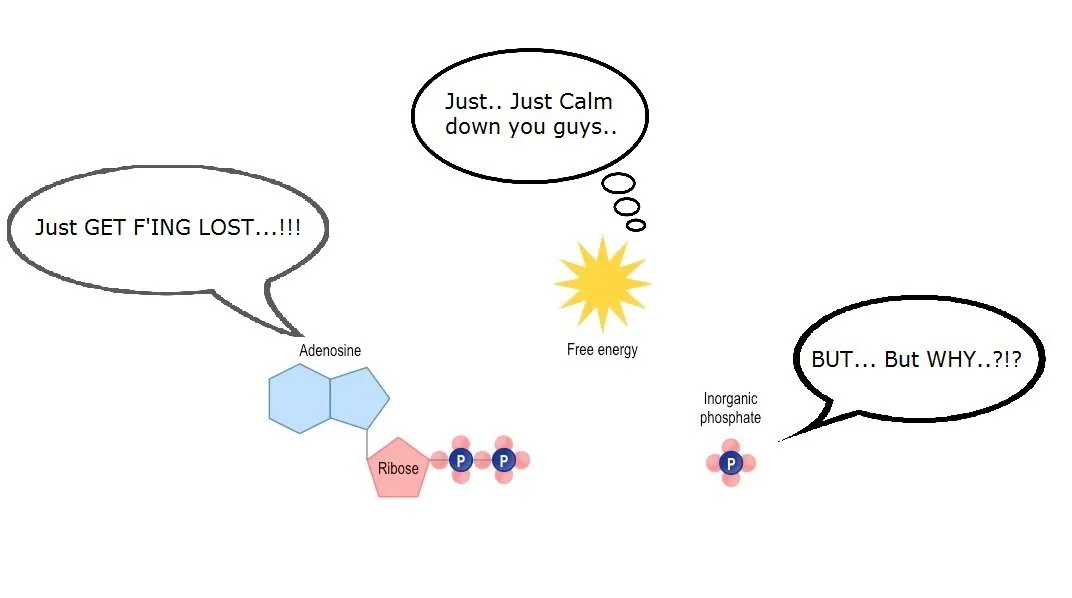
Image Credit + Edited by ME
This process of energy generation has some byproducts as well, like glucose and water. "Oxidization" is the process in which glucose combines with oxygen.
So this is the main reason why we need oxygen. Now in our atmosphere, the major gas is oxygen (20.95 %) after nitrogen (78.09 %), which generates another question as to why we don't breath nitrogen instead of oxygen.
Well, it'd be better to breath nitrogen, which would get rid of carbon dioxide, but then again, oxygen is more reactive. Nitrogen is very slow in reacting. If we breath nitrogen instead of oxygen, our cells could not generate the energy we need for our daily routine. Our cells harness this energy in a more controlled manner, simply put, it burns things with oxygen to get energy, more like a uncontrollably spreading fire.
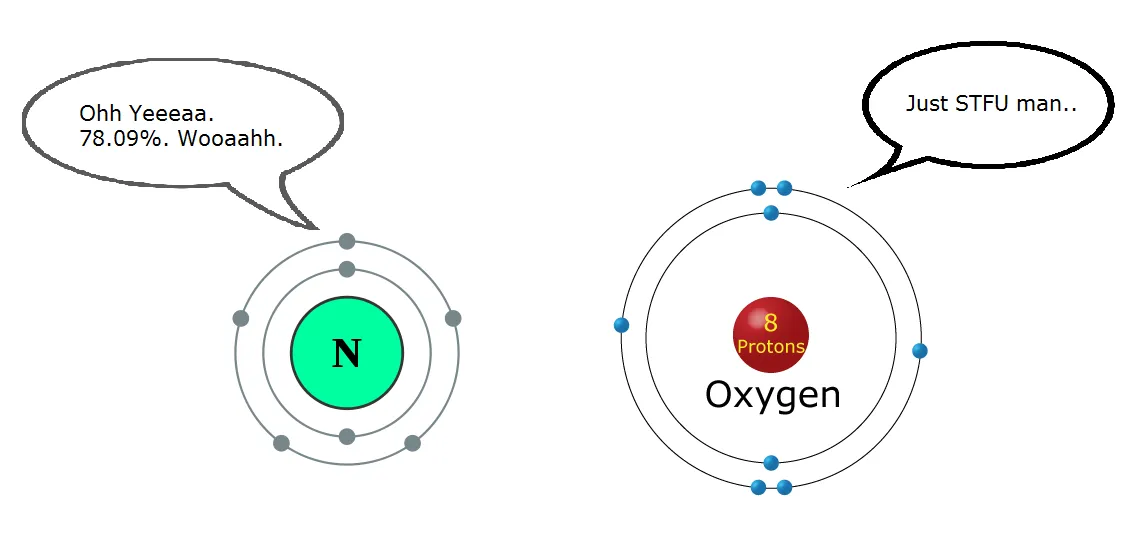
Image Credit + Edited by ME
I hope this answers the question in the easiest way possible. If you have any question, write down in the comments, and I'll try to cover it in my next article of this series.
Online References:
https://www.reference.com/science/humans-need-oxygen-live-d30657e8ef144fff
https://en.wikipedia.org/wiki/Cellular_respiration
https://answers.yahoo.com/question/index?qid=20090719210019AA0UQBH