Hello friends
I hope you are doing good in life.
Let's go directly towards the topic of the day.Answer of the question is simply the under water never get freezed.We all know about it. But the question is why do underwater never get frozen in a place like Antarctica.
Before getting into details we must know about a piaculliar feature of water. Do you know maximum density of water is at 4°c. If we increase temparature, the water will become less denser and if we go below 4°c, the water will also get less denser.The things with lesser density than water will sink in it.
We have to consider three things at the same time and they are volume ,temparature and density of water.Normally if anything is heated its volume increase as the temparature increase. But for water it is not always ture. We know water get freezed at 0°c and we also know ice have much volume than the same amount of water because with much volume the particle of water maintain more distance than that of water with less volume. With more distance between particles , the density of ice at 0°c will have lesser than that of water at 4°c.
Let's try to understand it with the help of volume, density and temparature chart of water as below:
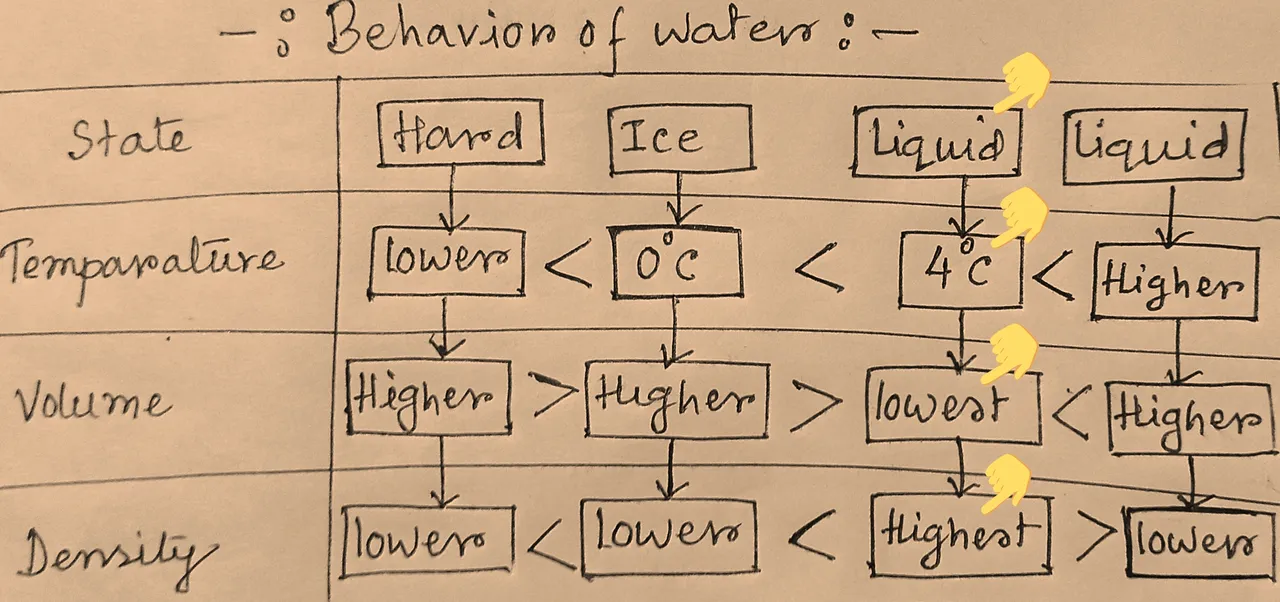 Hand made
Hand made
So what we got : water at 4°c ,it remains in liquid form, got the highest density and the lowest volume. This piaculliar feature saves marine life in cold places like Antarctica.This is fascinating indeed.
Let's discuss how does it happen.The enviornment become cooler and when the surface water reach 4°c and then it goes to 0°c and the again to minus temparature.The beauty of nature is the surface water at 4°c goes to the bottom of the water bodies as it got the maximum density and comparably the water with lower density and higher volume rises from the bottom and get cooled when temparature goes more lower than 4°c , its density decrease. With lower density on the top and the highest density at the bottom of water bodies can't replace its place. Because light water will never go to the bottom of the water.As water doesn't transfer heat , the lower water got the highest density forever, no matter the weather how much get cooled.
You may have doubt if water doesn't transfer heat or may have question how food in water get boiled then. There are three way to transfer heat.There methods are conduction, convection and radiation. Metals conduct heat , our enviornment gets heated by radiation and water follows the method convection to get raised in temparature.
Convection is a process of heating in which water particle moves after getting lighter by the heat and the comparably cool particle of water come down with higher density at the bottom. So when we cook food the water of bottom of a cooking utensil get heated first and then denser particle from above comes down with other things we cook. The process continues until the water reach the boiling point.
The conclusion
Water at the bottom of a water body in a place like Antarctica never get freezed because of unique feature of water and that is water at 4°C has the greatest density with lowest volume. This unique behavior of water helps marine life to survive in cold condition.This is the beauty of nature. It seems God himself told water to behave like that. Think otherwise what would happen to the life underwater if all of the water got freezed with the entire enviornment above the water.
I hope you have liked my article.
Thank you so much for stoping by.
Have a great day.
Regards: @th4488  Join my discord here.
Join my discord here.
The communities I am connected to from begining of my hive journey are as follows:
 Join TheTerminal discord here.
Join TheTerminal discord here.
 . Join indiaunited discord here.
. Join indiaunited discord here.
 Join ecency discord here.
Join ecency discord here.
Stay safe and stay healthy
