Hi Everyone,

In my post My Concerns about the Covid-19 Vaccines, I outlined several concerns I have about the Covid-19 vaccines. Governments and the mainstream media have promoted the Covid-19 vaccines as the only solution to ending the Covid-19 pandemic permanently. Any negative news or safety concerns are given very little exposure. When people attempt to share concerns or information that may indicate that the vaccines are not safe or might not be effective, they are likely to face some form of censorship. This is particularly true for social media such as Facebook, Twitter, and YouTube. Therefore, the information available is distorted in favour of vaccines. If information is distorted, people are less likely to be making informed decisions. Informed decisions regarding vaccination is important, as vaccination could have significant and possibly permanent impact on our lives.
My content is not subject to censorship, as I post on Hive through user interfaces such as PeakD and 3Speak. To be able to escape the possibility of censorship enables me to speak freely about various areas that are being restricted to a particular narrative on other social media. Unfortunately, Hive is relativity small. Therefore, most of my sources of information come from outside the Hive ecosystem. These sources may not be permanent (i.e. could be subject to some form of censorship). This will lead to some of my links to sources failing at some point. My sources that relate to data collection are more likely to remain, as they are less likely to be seen as controversial. However, some sites update regularly and may not display the exact same figures as they did when I accessed them. I normally include a date for this type of data.
Cost Benefit Analysis

Before continuing with this post, I strongly recommend that you read my post Economic Concepts – Cost Benefit Analysis (Steps Involved) as there are references to terminology, which are not adequately explained for someone who has limited knowledge of CBA.
In this post, I want to present a basic Cost Benefit Analysis (CBA) framework for vaccines with a focus on the Covid-19 vaccines. I believe a CBA framework would be valuable for assessing the costs and benefits of vaccines for the entire rollout as well as for different demographics. It is possible that vaccinations will be in the best interest of some groups of people and not others. However, we need some method of being able to gauge which groups these might be.
The best place to begin developing a specialised CBA framework is to use existing generic CBA frameworks. The basic structure of a CBA does not need to vary significantly. Below is a generic CBA framework, which I explained in my post Economic Concepts – Cost Benefit Analysis (Steps Involved) and in my Udemy CBA Course.
- Define the desired problem and outcome
- Define the base case
- Define the project case/s (Options)
- Identify benefits and costs
- Determine the referent group/s
- Determine key drivers of identified benefits and costs
- Formulate assumptions to enable quantification of benefits and costs
- Quantify benefits and costs
- Assign monetary value to identified benefits and costs
- Discount monetised benefits and costs
- Compare discounted benefits and costs
- Calculate economic measures and indicators
- Conduct sensitivity analysis
- Report the results of the CBA
In the context of Covid-19 vaccines, the CBA would be considered ex-post (i.e. occurring after the investment decision has been made. CBAs are normally ex-ante (i.e. occur prior to investment and used to determine if investment is viable). An ex-post CBA draws actual numbers (i.e. data reflecting what has happened) as well as projections (i.e. data that reflects what may happen). Ex-post CBAs are conducted after the investment decision but not necessarily at the end of the evaluation period. Ex-ante CBAs are completely forward looking; therefore, data used are estimates and projections. All benefits and costs that occur prior to the ex-ante CBA are considered sunk; therefore, not included in the CBA.
Define the desired problem and outcome
The problem is serious illness and possibly death caused by Covid-19. The desired outcome is to reduce the number of people seriously ill and dying to an acceptable level (e.g. similar to a seasonal flu).
Define the Base Case
In the base case, people are not vaccinated. People need to rely on their own actions (e.g. wear masks in crowded enclosed areas, social distance from vulnerable people, wash hands more regularly) and natural immunity (e.g. boost natural immunity by taking vitamins, eating healthier, and exercising) to protect themselves against Covid-19. Existing pharmaceutical products such as Hydroxychloroquine can be considered as a base case response.
An alternative base case could be to assume that some people have been vaccinated and/or have gained some immunity to Covid-19. This base case would be useful for determining if a particular group of people should be vaccinated if a vaccine is available. As this is the base case, it would be assumed that this particular group of people are not vaccinated.
Define the Project Case/s (Options)
The main project case would be the development and distribution of a Covid-19 vaccine. The extent of this distribution will depend on demand. The demand will be higher if Governments rather than individuals buy the vaccines. It is typical in many countries that Governments buy the vaccines using taxpayer money and then distribute the vaccine for no additional charge. People who opt not to take the vaccine are indirectly subsidizing the people that take the vaccine. Hence, people are more likely to take the vaccine.
An alternative project case could be constructed for particular groups of people deciding whether to be vaccinated. This project case would exclude distribution and development costs, as these would have occurred in the alternative base case. If the Government had bought the vaccines, cost per individual vaccine would not be relevant. This alternative project case would be compared with the alternative base case described in the previous section.
It would be unwise to consider a vaccine response as the only medical approach to reducing the harm caused by Covid-19. Other options must be included as part of the CBA. These would include the development of treatments and cures. The outcome is not to eliminate Covid-19 but to reduce the damage it causes to an acceptable level.
Identify Benefits
For the main base and project cases, the benefits would be the reduction in costs to personal health (i.e. reduced illness and death). To calculate the benefit from reduced illness and death, we need to calculate cost of illness and death in the base case and the project case/s. The benefits would be calculated by subtracting the project case costs of personal health from the base case costs of personal health.
In the main base case, costs of illness and death would relate directly to Covid-19. This would be illness and death occurring during infection as well as any lasting effects such as long Covid. However, in the project case, costs of illness and death would also need to include any adverse reactions to the vaccine. The project case would also need to consider any negative effects of virus mutation induced by the vaccine. If the vaccine significantly reduces transmission, herd immunity could be considered as a benefit. This would most likely be reflected as a larger reduction in probability of infection (explained in quantification section of post).
For the alternative base and project case/s, mutation of the virus and herd immunity should be considered in base and project cases. This is because we are focusing on a particular group of people with the assumption other groups are being vaccinated.
For options such as cures and treatments. The main base case should be used but the project case/s will be different. The success rates at reducing illness and death must be included along with any possible negative effects these cures and treatments cause (assuming this information is available).
Identify Costs
For the main base and project cases, the costs would be the costs of developing, supplying and distributing the vaccine. For the alternative base and project case/s for particular groups of people, most costs are expected to occur in the base case, where the group would be required to seek non-vaccine options if they became infected. For the base case and project case/s for the non-vaccine options (i.e. cures and treatments), the costs would be the costs of developing, supplying and distributing the cures and treatments (very similar to the vaccine main case).
Determine the referent group
The referent group would be everyone. Anyone could contract Covid-19 and anyone could become ill or even die. However, not everyone has the same level of risk. Elderly people with multiple serious conditions have a much higher chance of becoming seriously ill and dying. Young people with no serious medical conditions have very low chances of falling seriously ill and almost no chance of dying. Therefore, the referent group needs to be divided into many sub-referent groups. These sub-referent groups could be divided based on age, existing serious medical conditions, and sex (if data suggests differences between sexes).
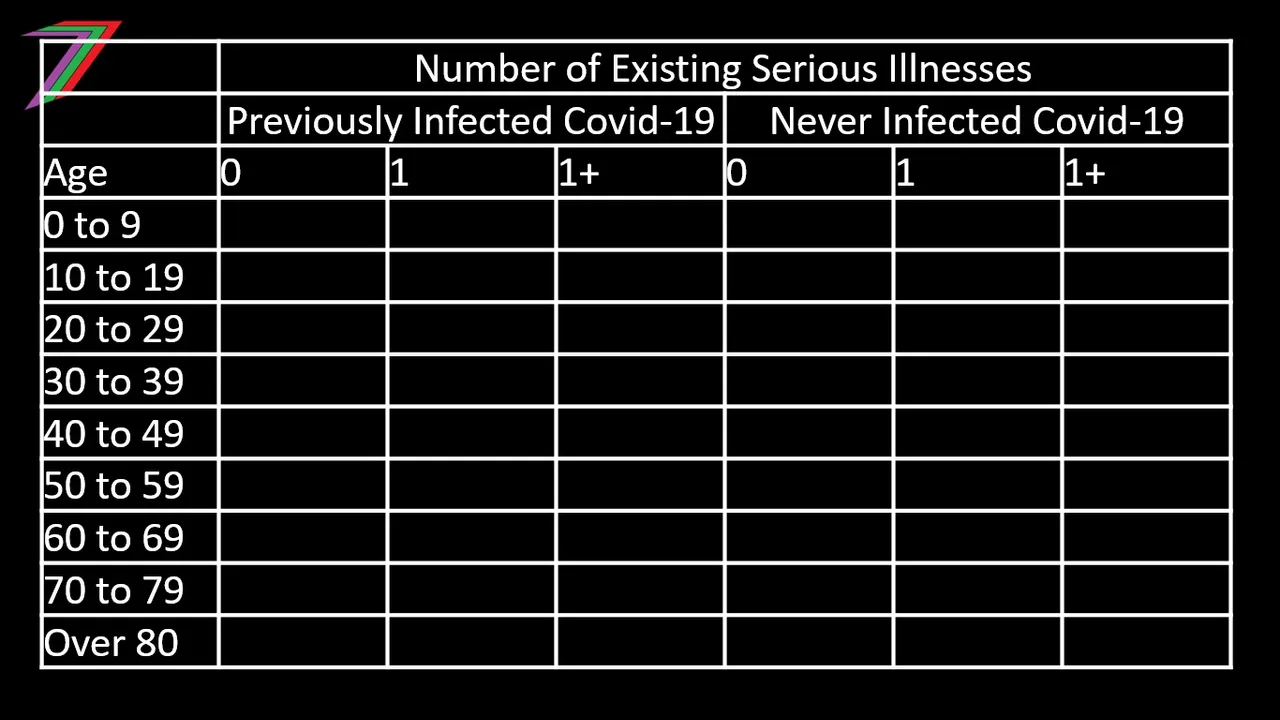
We could argue that the sub-referent groups could be divided further based on previous infection of Covid-19. People that have had Covid-19 should have developed some immunity to the virus. This would reduce their chance of catching Covid-19 again. If they do catch it, they are less likely to become seriously ill or die. Table 1 outlines the possible sub-referent groups that could be used for the CBA.
Table 1: Possible Sub-referent Groups
Determine key drivers of identified benefits and costs
The key drivers for benefits can be divided into two main groups. The first group relates to the probabilities of infection, serious illness, and death. The second group relates to health costs of serious illness and death. Higher probabilities and health costs in the base case signifies a more serious problem. Therefore, the potential benefits of a vaccine would be higher.
For these potential benefits to be realised, the vaccine needs to reduce the probability of serious illness and death significantly. The probability and health costs from negative effects of the vaccine are also an important driver of benefits. The benefits of the vaccine will be higher if there is a very low probability of negative effects and if negative effects do occur, they are minor.
There are numerous key drivers of costs. Some of drivers could include:
- duration of research and development
- duration of testing and trialling
- the cost of manufacturing the vaccine
- the cost of storing the vaccine
- the cost of distributing the vaccine
- capacity and capability to produce the vaccine
- ability to finance initial outgoings
- safety standards and requirements
- availability of required expertise
There is also substantial risk in developing a vaccine, as the initial outlay is high and there is no guarantee a vaccine will ever be developed. Failure to develop a vaccine should be considered a cost without any benefit. In the case of Covid-19, vaccines have been developed and approved. Therefore, this risk has been removed for this example.
Formulate assumptions to enable quantification of benefits and costs
Even in an ex-post CBA, assumptions are still likely to be required. Assumptions are needed to construct the base and project case/s; see earlier paragraph. Assumptions were used to determine the referent and sub-referent groups. Assumptions are needed when data is incomplete. Probabilities of infection will be difficult to determine. Different people have different risks depending on factors such as lifestyle, occupation, culture, and extent of precautions taken. All people within each sub-referent group would be assumed the same level of risks. Different existing illnesses pose different risks but the vast number of different illnesses makes it impossible to distinguish between them. Therefore, all serious existing illnesses would be treated the same. Collected data could vary in terms of reliability. However, it is difficult to determine the extent of this difference. Data can be adjusted if problems can be identified. Otherwise, all data would be treated as equally reliable. Health costs would be determined based on existing research. The value of statistical life can be used for deaths. These values can be adjusted for different age groups. Health costs for serious illness will be more difficult to determine. Average duration in hospital and time taken off work can be considered. This can be adjusted for different age groups.
Quantify benefits and costs
The number of people that have had serious illness or have died because of Covid-19 needs to be quantified in the base case/s and project case/s. Projections for the number of people who will suffer serious illness or will die will also need to be quantified. The number of people that have suffered serious adverse reactions to the vaccines needs to be quantified. If possible, the quantification should also consider different variants of Covid-19.
The benefits should be quantified for each sub-referent group, as probabilities of infection, serious illness, and death varies for each group. The benefits should be presented as both individual figures and aggregate figures for the group. A more detailed presentation of results adds both transparency and rigour to the analysis. It also enables more flexibility for sensitivity testing.
Before benefits can be quantified data needs to be collected. The data should relate to the following areas for each identified sub-referent group.
- Probability of contracting Covid-19
- Probability of serious illness caused by Covid-19
- Probability of death caused by Covid-19
- Probability of long Covid.
- Probability of serious illness caused by vaccine.
- Probability of death caused by vaccine.
- Probability of vaccine resistant new strain.
- Probability of contracting new strain of Covid-19.
- Population
Once the data has been collected and adjusted based on assumptions, the benefits can be quantified. The following formulae have been suggested for a sample sub-referent group.
Individuals:
% Reduction in serious illness = % Serious Illness (Base Case) - % Serious Illness (Project Case)
% Reduction in serious illness = (PC19(BC) × PI(BC)) - (PC19(PC) × PI(PC))
% Reduction in Covid-19 deaths = % Covid deaths (Base Case) - % Covid deaths (Project Case)
% Reduction in Covid-19 deaths = (PC19(BC) × PD(BC)) - (PC19(PC) × PD(PC))
% Reduction in long Covid = % Long Covid (Base Case) - % Long Covid (Project Case)
% Reduction in long Covid = (PC19(BC) × PL(BC)) - (PC19(PC) × PL(PC))
% Serious Immediate Adverse Reaction
% Serious Long term Adverse Reaction
% Vaccine Death
% Reduction in serious illness (variant) = % Serious Illness (Base Case) - % Serious Illness (Project Case)
% Reduction in serious illness (variant) = (PC19V(BC) × PIV(BC)) - (PC19V(PC) × PIV(PC))
% Reduction in Covid-19 deaths (variant) = % Covid deaths (Base Case) - % Covid deaths (Project Case)
% Reduction in Covid-19 deaths (variant) = (PC19V(BC) × PDV(BC)) - (PC19V(PC) × PDV(PC))
Where:
BC – Base Case
PC – Project Case
PC19 – Probability of infection
PI – Probability of serious illness
PD – Probability of death
PL – Probability of long Covid
PC19V – Probability of infection (variant)
PIV - Probability of serious illness (variant)
PDV – Probability of death
Whole Group:
Reduction in serious illness = No. Serious Illness (Base Case) - No. Serious Illness (Project Case)
Reduction in serious illness = (PC19(BC) × PI(BC) × PGX) - (PC19(PC) × PI(PC) × PGX)
Reduction in Covid-19 deaths = No. Covid deaths (Base Case) - No. Covid deaths (Project Case)
Reduction in Covid-19 deaths = (PC19(BC) × PD(BC) × PGX) - (PC19(PC) × PD(PC) × PGX)
Reduction in long Covid = No. Long Covid (Base Case) – No. Long Covid (Project Case)
Reduction in long Covid = (PC19(BC) × PL(BC) × PGX) - (PC19(PC) × PL(PC) × PGX)
Serious Immediate Adverse Reaction = % Serious Immediate Adverse Reaction × PGX
Serious Long term Adverse Reaction = % Serious Long term Adverse Reaction × PGX
Vaccine Death = % Vaccine Death × PGX
Reduction in serious illness (variant) = No. Serious Illness (Base Case) – No. Serious Illness (Project Case)
Reduction in serious illness (variant) = (PC19V(BC) × PIV(BC) × PGX) - (PC19V(PC) × PIV(PC) × PGX)
Reduction in Covid-19 deaths (variant) = No. Covid deaths (Base Case) – No. Covid deaths (Project Case)
Reduction in Covid-19 deaths (variant) = (PC19V(BC) × PDV(BC) × PGX) - (PC19V(PC) × PDV(PC) × PGX)
Where:
PGX = Population in Group X (Sample Group)
All Groups:
Total reduction in serious illness = Sum of reduction in serious illness for all groups
Total reduction in Covid-19 deaths = Sum of reduction in Covid-19 deaths for all groups
Total Serious Immediate Adverse Reaction = Sum of Serious Immediate Adverse Reaction for all groups
Total Serious Long Term Adverse Reaction = Sum of Serious Long Term Adverse Reaction for all groups
Total Vaccine Deaths = Sum of Vaccine Deaths for all groups
Total reduction in serious illness (variant) = Sum of reduction in serious illness (variant) for all groups
Total reduction in Covid-19 deaths (variant) = Sum of reduction in Covid-19 deaths (variant) for all groups
Note: Many of the formulae presented could be simplified. For the purpose of this post they have not been simplified. This is because it is important to show each component of the equation. Simplification could make the equations less intuitive.
All of the above formulae could be adjusted for the percentage of the population vaccinated. This percentage could be used to determine if vaccination has an impact (i.e. positive or negative) on herd immunity. The implications of herd immunity could be a determinant for vaccination at an individual level.
Benefits will need to be calculated for each year in the evaluation period. The evaluation period should equate to approximately the duration Covid-19 would remain a health threat without intervention. This should be approximately equivalent to how long it would take society to gain sufficient immunity to reduce Covid-19 to a reasonably threat (e.g. similar to seasonal flu).
Benefits for alternatives to the vaccine such as cures and treatments should be calculated using similar formulae as described for the vaccine.
Cost are most likely to be quantified and monetised at the same time; see next section for more detail.
Assign monetary value to identified benefits and costs
After the probabilities and numbers of people have been estimated, a monetary value can be assigned to determine the benefits and possibly disbenefits of the vaccine. Value of statistical life can be applied to deaths and could be adjusted for each group based on age. Values for serious illness can be determined based on suffering, costs of medication and treatment, and loss of income. Values for serious illness are likely to vary for each group and possibly for each variant of the virus. Values for adverse reactions will depend on the expected duration of the adverse reaction and could be determined using similar variables used for determining values for serious illness.
Before benefits can be monetised, data needs to be collected. For the purpose of monetisation, data is normally sourced from research groups that have determined costs based on studies. For example, value of life is determined by studies using willingness-to-pay and cost of injury or illness is determined using a human capital approach. Below are the cost data required to monetise benefits for this CBA.
- Cost of serious illness caused by Covid-19.
- Cost of death caused by Covid-19.
- Cost of long Covid.
- Cost of serious illness caused by vaccine.
- Cost of serious illness caused by new strains of Covid-19.
Using the cost data described above and the quantified benefits, it is possible to monetise benefits for the CBA. The following formulae for mortising benefits have been suggested for a sample sub-referent group.
Individuals:
Benefit from reduced chance of serious illness = % Reduction in serious illness × Cost of Serious Illness
Benefit from reduced chance of death = % Reduction in Covid-19 deaths × Value of Life
Benefit from reduced chance of long Covid = % Reduction in long Covid × Cost of Long Covid
Disbenefit from chance of serious immediate adverse reaction = % Serious Immediate Adverse Reaction × Cost of Immediate Adverse Reaction
Disbenefit from chance of serious long term adverse reaction = % Serious Long term Adverse Reaction × Cost of Long term Adverse Reaction
Disbenefit from chance of various death = % Vaccine Death × Value of Life
Benefit from reduced chance of serious illness (variant) = % Reduction in serious illness (variant) × Cost of Serious Illness (variant)
Benefit from reduced chance of death variant = % Reduction in Covid-19 deaths (variant) × Value of Life
Whole Group:
Benefit from reduced No. of serious illness = Reduction in No. serious illness × Cost of Serious Illness
Benefit from reduced No. Covid-19 deaths = Reduction No. Covid-19 deaths × Value of Life
Benefit from reduced long Covid = Reduction No. long Covid × Cost of Long Covid
Disbenefit from serious adverse reactions = No. Serious Immediate Adverse Reaction × Cost of Serious Immediate Adverse Reaction
Disbenefit from serious adverse reactions = No. Serious Long-term Adverse Reaction × Cost of Serious Long term Adverse Reaction
Disbenefit from vaccine death = No. Vaccine Death × Value of Life
Benefit from reduced No. of serious illness (variant) = Reduction in No. serious illness (variant) × Cost of Serious Illness (variant)
Benefit from reduced No. Covid-19 deaths (variant) = Reduction No. Covid-19 deaths (variant) × Value of Life
All Groups:
Total benefit from reduction in serious illness = Sum of benefits from reduction in serious illness for all groups
Total benefit from reduction in Covid-19 deaths = Sum of benefits from reduction in Covid-19 deaths for all groups
Total benefit from Serious Immediate Adverse Reaction = Sum of benefits from Serious Immediate Adverse Reaction for all groups
Total disbenefit from Serious Long Term Adverse Reaction = Sum of disbenefits from Serious Long Term Adverse Reaction for all groups
Total disbenefit from Vaccine Deaths = Sum of disbenefits from Vaccine Deaths for all groups
Total benefit from reduction in serious illness (variant) = Sum of benefits from reduction in serious illness (variant) for all groups
Total benefit from reduction in Covid-19 deaths (variant) = Sum of benefits from reduction in Covid-19 deaths (variant) for all groups
The costs of developing the vaccine should be sourced from the manufacturers. If costs cannot be sourced from the manufacturers they could be sourced from Governments that have purchased the vaccines. The costs for distributing the vaccines should be sourced from agencies responsible for distributing the vaccine (e.g. National Health Service).
Discount monetised benefits and costs
Discounting should be conducted as per standard CBA. A base year needs to be established. All costs and benefits should be discounted to that base year. A consistent discount rate should be applied to all costs and benefits. This discount rate should be equivalent to a standard social discount rate used for social projects. Sensitivity testing should be conducted with at least one higher and one lower discount rate.
Compare discounted benefits and costs
Benefits and costs should be compared at both disaggregate and aggregate levels. The benefits and costs should be compared at a group level (disaggregate) first. This will determine if the group has a net benefit or disbenefit from taking the vaccine. Two different approaches can applied to comparing benefits and costs at the aggregate level. The first approach involves aggregating benefits and costs for all groups. The second approach involves only aggregating benefits and costs for groups that have a net benefit from taking the vaccine. The second approach could be useful for determining the impact on herd immunity if some groups do not take the vaccine. The second approach could be used to modify the calculation of the impact the vaccine has on different variants of the virus. The aggregate figures could be further adjusted downwards for an assumed percentage of people in groups with a net benefit who opt not to take the vaccine.
For an ex-ante CBA, the costs of production could be adjusted to meet the demand suggested by the analysis of the net benefits for each group. For example, if it is predicted that 40% of the population will be vaccinated, the costs of production can be reduced to meet that level rather than if it is assumed 100% of the population are to be vaccinated.
The benefits and costs of the vaccine should also be compared with alternative approaches such as cures and treatments. This could involve a direct comparison between alternative approaches and the vaccine or an approach that involves both the vaccine and alternative approaches; If a vaccine is not 100% effective, cures and treatments will be required.
Calculate economic measures and indicators
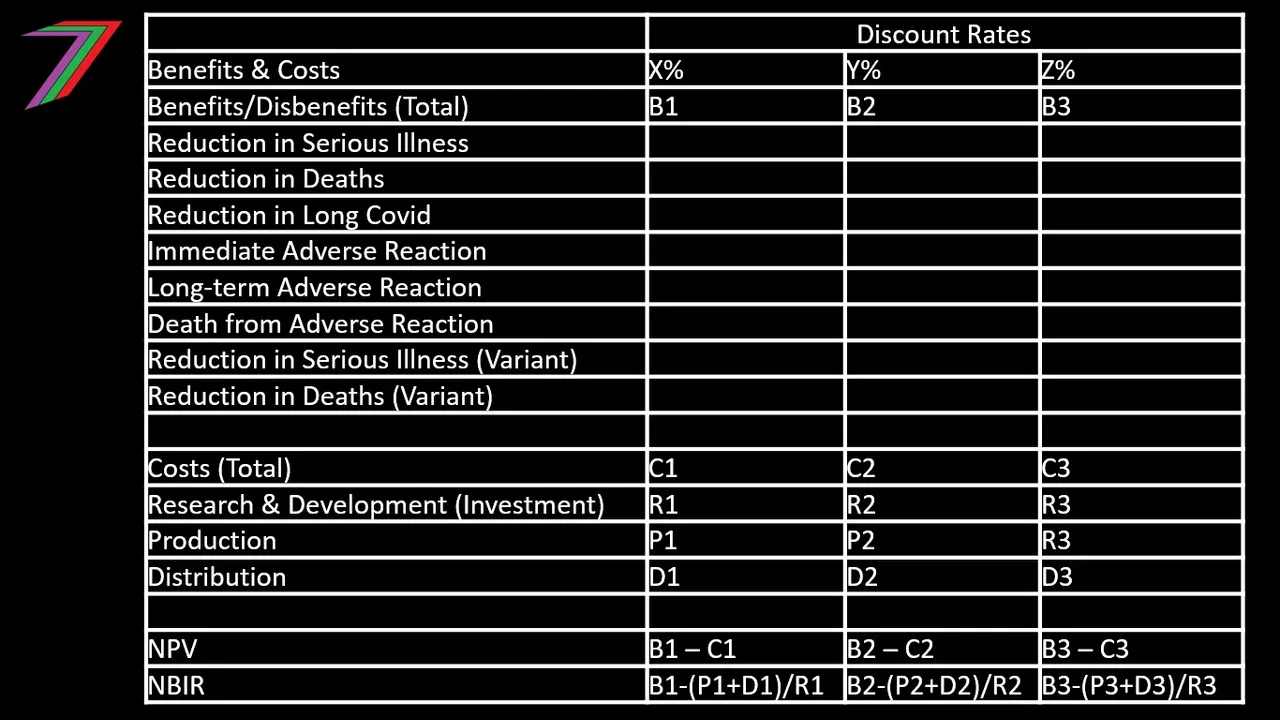
A CBA typically uses economic indicators such as Net Present Value (NPV), Benefit Cost Ratio (BCR), Net Benefit Investment Ratio (NBIR), incremental ratios for options (e.g. IBCR or INBIR), and Internal Rate of Return (IRR). All these measures are relevant to a vaccine CBA. The NPV and incremental ratios are likely to be the most relevant. The NPV will determine if vaccine development is viable as well as the extent of the net benefit or disbenefit of a vaccine program. The incremental ratios are critical for comparing a vaccine approach to other forms of medical treatments.
Conduct sensitivity analysis
Sensitivity analysis would be essential for a vaccine CBA. This is because of the level of uncertainty around many areas. These include:
- Expected uptake of vaccine.
- Predicting efficiency of vaccine against new variants and mutations.
- Predicting how the vaccine may induce mutations in the virus.
- Extent of adverse reactions, particularly long-term adverse reactions.
- The type of long-term adverse reactions.
- The duration of the pandemic in the absence of vaccines.
- Types of Government action.
Sensitivity tests should be conducted for all of the above areas of uncertainty. Three scenarios can be set up for many of them. For example, there could be low, medium, and high uptake scenarios of the vaccine. Sensitivity testing can be applied to the evaluation period to assess how a long pandemic would compare to a short pandemic. Government actions such as restrictions and lockdowns could be considered in a sensitivity test (see, later section for a more detail explanation of this approach).
Sensitivity tests should be conducted and presented for sub-referent groups and at the aggregate level. Changing of variables or parameters for a group could lead to the net benefits switching from positive to negative or vice versa. This would lead to a change in expected uptake as well as any other changes in variables and parameters at the aggregate level. It is also important to determine how sensitivity varies from group to group. Different groups will be sensitive to different inputs. For example, older age groups are likely to be more sensitive to vaccines that produce a particular type of adverse reaction.
The sensitivity analysis could be enhanced further with Monte Carlo simulation or construction of distributions of benefits and costs through other means. This would enable the calculation of probabilities that the vaccine produces net benefits for each sub-referent group as well as the whole population. It is possible that a percentage confidence in success is more important than simply achieving a positive NPV. An average NPV and NBIR across all scenarios and sensitivities would also be calculated for inclusion in the report.
Report the results of the CBA
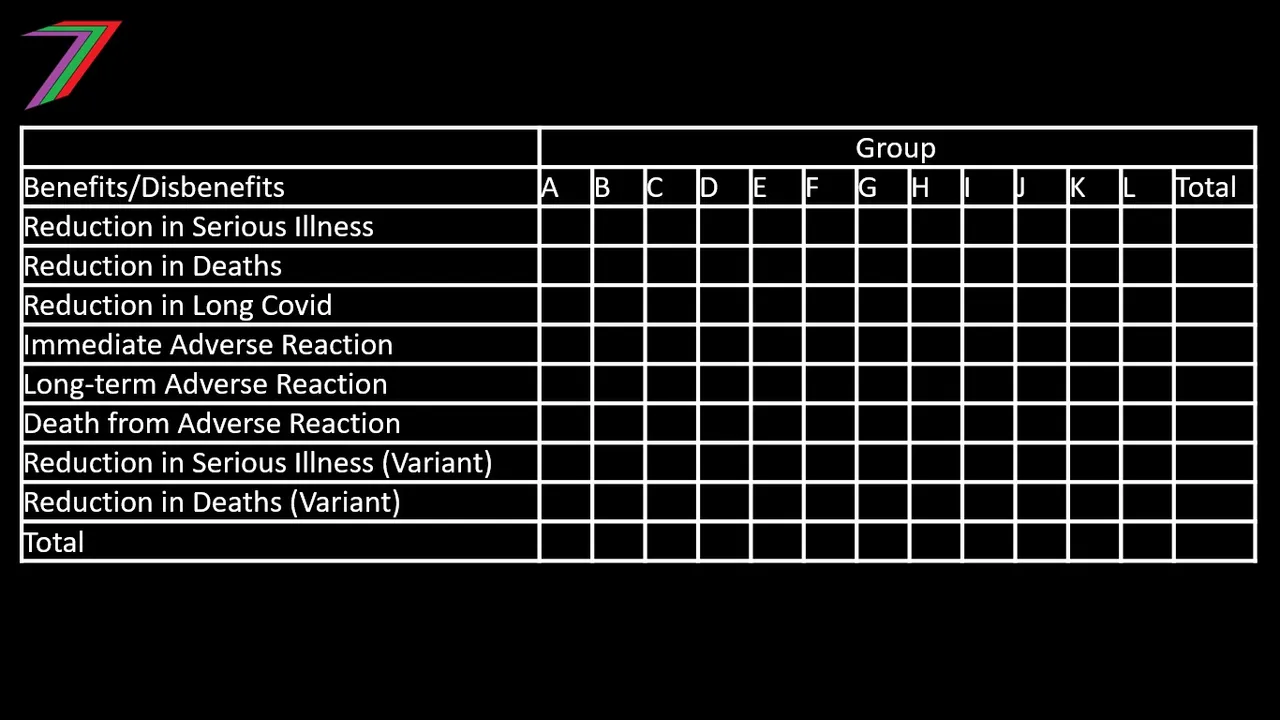
The CBA report for a vaccine would need to be highly comprehensive. It would need to explain the results for each sub-referent group. See Table 2 for how the results of each group could be presented.
Table 2: Benefits and Disbenefits of each group
The report would need to provide interpretations of the results as well as why each group should or should not be recommended to take the vaccine. The report would need to state the impact on the aggregate results if particular groups are recommended to be excluded. The report should also discuss how the results could be used to help individuals determine if they are going to take the vaccine. The options analysis of the CBA will be important. Results should not only be based on comparisons with the base case (i.e. rely purely on their own actions) but also with comparisons with other project cases. These other project cases would represent alternative medical approaches such as cures, treatments or even modifications to existing treatments.
The rest of the report should closely resemble a standard CBA report. The key elements of a CBA report are summarised below.
- Executive Summary
- Introduction
- Base Case/s
- Project Case/s
- Assumptions
- Methodology
- Summary of Input Data
- Costs
- Benefits
- Summary of Costs and Benefits
- Results
- Sensitivity Analysis
- Additional Supporting Analysis
- Recommendation
- Conclusion
- References
- Appendices
Why is Government action not considered in the base case?

The proposed CBA framework is comprehensive in terms of detail required to conduct a CBA for a vaccine. A notable exclusion from the main base case is Government actions regarding Covid-19. Government actions such as restrictions and lockdowns are occurring in the absence of vaccines; therefore, arguably should be considered as part of the base case. However, Government actions such as restrictions and lockdowns are still occurring with the rollout of the vaccine. Hence, we could argue, to a certain extent, the impact of Government actions in the base and project case would cancel each other out. A stronger argument for excluding Government actions is the level of complexity they would add to the CBA. Restrictions imposed by Governments has extremely broad implications across the whole economy and beyond. A CBA cannot be equipped to deal with such complexity. Instead, a general equilibrium analysis would be a more appropriate approach. The possible impact of Government actions could be simplified as part of the sensitivity analysis or could be included as a qualitative discussion in the CBA report.
Conclusion

Conducting a CBA for a vaccine is challenging, as there are many unknown elements. The number of the unknown elements can be greatly reduced by conducting an ex-post CBA (i.e. after the decision to invest has been made). The ex-post CBA would not be used to determine if the analysed vaccine should be developed but would be very useful in other ways.
- It could determine if particular sub-referent groups of people should be recommended to take or not take the vaccine.
- It could help people decide if they should take the vaccine regardless of the sub-referent group they belong to, as they will be presented with the risks of vaccinating compared to not vaccinating.
- It could help determine how much of the vaccine should be produced in future years.
- It could provide an objective measure of the success of the vaccine compared to alternative medical approaches.
- It could help determine if vaccines for similar viruses could be viable.
The CBA framework would need to be tested with an actual CBA. It is quite likely that some of the data is going to be difficult to obtain or verify which will lead to more assumptions. These assumptions would then require more rigorous testing in the sensitivity analysis. It is also possible that the vaccines will have other benefits or disbenefits not discussed in this post. Research is always ongoing. The CBA framework should be modified to include any new findings.
More posts

If you want to read any of my other posts, you can click on the links below. These links will lead you to posts containing my collection of works. These 'Collection of Works' posts have been updated to contain links to the Hive versions of my posts.
My New CBA Udemy Course
The course contains over 10 hours of video, over 60 downloadable resources, over 40 multiple-choice questions, 2 sample case studies, 1 practice CBA, life time access and a certificate on completion. The course is priced at the Tier 1 price of £20. I believe it is frequently available at half-price.
Future of Social Media