This is part of an on-going series of posts that are going to explain how to produce or synthesize certain useful chemicals to allow one to acquire them without going through chemical supply vendors whether that be to reduce the cost of acquiring chemicals or to hide the purchases from prying eyes.
Prelude
Nitric acid cannot be distilled beyond roughly 68% meaning that any nitric acid above 68% has to be formed through other methods. This method will be able to produce really high purity (>99%) which can be useful for a number of purposes. For those that do not know, an azeotrope is a solution composed of two or more liquids where once a certain concentration is met the boiling point of both liquids becomes the same meaning simple distillation can no longer be used to separate the liquids. This is why you cannot use distillation to get super high concentrations of nitric acid, though you can get up to 68% concentrated by weight.
Red Fuming Nitric Acid is abbreviated as RFNA
White Fuming Nitric Acid is abbreviated as WFNA
Required Materials
- Sodium Nitrate salt (powder)
- Azeotropic Sulphuric Acid (distillation explanation here)
Recommended Materials
- Round bottom boiling flask
- Condenser
- Hot plate
Where to buy Sodium Nitrate
Unlike the sulphuric acid that is present at any hardware store it seems, sodium nitrate is a little bit more difficult to acquire at a suitable purity. Although you could purify it from certain types of fertilizers, it would be far easier to buy it from somewhere that sells materials for curing and smoking meats as sodium nitrate salts are used to cure meat. You may be able to ask local butchers if they use it and if so where they source it. This may allow you to bypass ordering through a chemical supply vendor as if you are ordering from a chemical supply vendor then it will probably be easier and cheaper just to buy FNA than to by nitrate salts.
Process
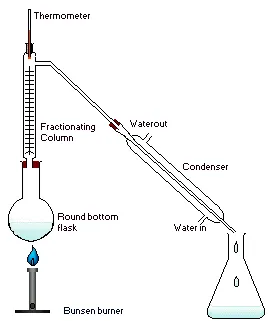
Setup a distillation setup similar to as shown to the left. However before you connect the boiling flask (round bottom in this image) you should add your sodium nitrate salt. Then right before connecting the flask add a slight excess of the stoichiometric amount of concentrated sulphuric acid, then connect everything up.
Apply heat to the boiling flask. The heating should be maintained around 83oC which is easiest done, in my opinion, with an oil bath or hot water bath though if you have a heating mantle that can maintain temperature then that can work too.
Collect your distillate until almost all to all of the sulphuric acid has reacted. Sodium Sulphate will remain in the reaction vessel. Do not be concerned if it is fuming red gas, this is just nitrogen dioxide contamination and is the reason its called RFNA. If left to site in a cool environment it will eventually fume off and convert to WFNA.
Balanced Equation
NaNO3(s) + H2SO4(conc) → HNO3(vapour) + NaHSO4(s)
Warning
Common gloves used in lab settings are nitrile gloves. Nitrile gloves are not adequate at protecting against RFNA or WFNA. Nitrile gloves offer some protection against azeotropic nitric acid but if you are working with nitric acid then using vinyl gloves will offer superior protection at a minimal increase in price. With other acids, if you spill nitric acid on your skin then wash it off immediately. Like sulphuric acid, nitric acid does produce an exothermic reaction when dissolved in water so it would be better to have a cold water bath than to just spray water on the affected areas. Note that it is normal for nitric acid to stain skin a red-orange colour. Do not panic if you spill nitric acid on yourself, calmly clean it off.
Video
Uses of RFNA and WFNA
Besides being useful for certain chemical reactions, FNA is often used to as an oxidizing agent in rocket propellants as it can form a number of hypergolic propellants (propellants that will spontaneously combust). FNA is often also used in the explosives industry to make explosive compounds as the nitric acid is used to deposit nitrogen groups onto base compounds and due to how much energy gets released from the formation of molecular dinitrogen it can be used to make very powerful explosives and high explosives. In future posts I might go into more depth of why nitrogen is used in so many explosives and I will definitely go into more uses of both nitric acid and fuming nitric acid.
Experiment
This is a fun little experiment where all you need are 4 things: Copper, WFNA, dilute nitric acid (I find a 20%-30% molar concentrations to be a relatively good concentration for the experiment), and azeotropic nitric acid (and glassware I guess). Take 3 beakers and add a piece of copper to each. In one beaker with copper add some dilute nitric acid. In the second beaker add some azeotropic nitric acid. In the third beaker add some fuming nitric acid to the copper.
Why this is cool is the dilute nitric acid will change the solution to a nice blue-green colour as the copper dissolves, the azeotropic nitric acid will fume nitrogen dioxide and eat away at the copper in minutes in a violent eruption, and the fuming nitric acid will barely even be able to react with the copper.
Explanation: The dilute nitric acid will slowly attack the copper producing copper(II) nitrate which is soluble in water and will make it go from a green to a nice blue colour, it also produces nitrogen dioxide but because the reaction is slow it shouldn't have enough gas to really impede the colour of the water. The azeotropic nitric acid (68%) will react much faster producing significantly more nitrogen dioxide and will react far more violently. However the odd thing is the fuming nitric acid wont really react all that much. This is because, to my knowledge, the copper(II) nitrate isn't being dissolved into the water or nitric acid as there isn't really a lot of water present. This stops the nitric acid from reacting with the copper and will instead change the copper to be greenish-blue colour. This reaction is called passivation and I will go more in depth on this.
Sources
Legal Notice
This is intended for educational purposes only. Do not try this at home without proper safety equipment and knowledge of what you are doing. I am not responsible for what you do with the information divulged in this series.
